Levosimendan
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Simdax |
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | IV |
| ATC code | C01CX08 (WHO) |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 85% (oral) |
| Protein binding | 97–98% |
| Metabolism | Extensive hepatic |
| Biological half-life | ~1 hour (levosimendan), 75–80 hours (metabolites) |
| Excretion | urine (54%), feces (44%) |
| Identifiers | |
| |
| CAS Number |
141505-33-1 |
| PubChem (CID) | 3033825 |
| DrugBank |
DB00922 |
| ChemSpider |
2298414 |
| UNII |
C6T4514L4E |
| KEGG |
D04720 |
| ChEBI |
CHEBI:50567 |
| ChEMBL |
CHEMBL313136 |
| ECHA InfoCard | 100.189.828 |
| Chemical and physical data | |
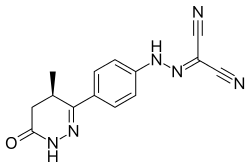
| Formula | C14H12N6O |
| Molar mass | 280.28 g/mol |
| 3D model (Jmol) | Interactive image |
| |
| |
| | |
Levosimendan (INN) /ˌliːvoʊsaɪˈmɛndən/ is a calcium sensitiser used in the management of acutely decompensated congestive heart failure. It is marketed under the trade name Simdax (Orion Corporation).
Mechanism of action
Levosimendan is a calcium sensitizer — it increases the sensitivity of the heart to calcium, thus increasing cardiac contractility without a rise in intracellular calcium. Levosimendan exerts its positive inotropic effect by increasing calcium sensitivity of myocytes by binding to cardiac troponin C in a calcium-dependent manner. It also has a vasodilatory effect, by opening adenosine triphosphate (ATP)-sensitive potassium channels in vascular smooth muscle to cause smooth muscle relaxation. The combined inotropic and vasodilatory actions result in an increased force of contraction, decreased preload and decreased afterload. Moreover, by opening also the mitochondrial (ATP)-sensitive potassium channels in cardiomyocytes, the drug exerts a cardioprotective effect.[1]
Clinical use
Indications
Levosimendan is indicated for inotropic support in acutely-decompensated severe congestive heart failure.
Some of the Phase III studies in the extensive clinical program were the trials LIDO (200 patients), RUSSLAN (500), CASINO (250), REVIVE-I (100), REVIVE-II (600) and SURVIVE (1350). In total, the clinical data base includes more than 3500 patients in Phase IIb and III double-blind studies.[2]
In the SURVIVE study, despite a reduction in plasma B-type natriuretic peptide level in patients in the levosimendan group compared with patients in the dobutamine group, levosimendan did not significantly reduce all-cause mortality at 180 days.[3] However, the drug was proven to be superior to dobutamine for treating patients with a history of CHF or those on beta-blocker therapy when they are hospitalized with acute decompensations.[3]
In a meta-analysis of randomized controlled studies by Landoni et al. levosimendan is shown to reduce mortality and hospitalization.[4]
Licensing status
The Orion Corporation originally developed levosimendan and applied for a new drug application in 1998 in the U.S. However the Food and Drug Administration (FDA) requested further trials be conducted and Orion withdrew the application in November 1999. Initially, Orion obtained the approval to market the drug in Sweden in 2000.[5] Since then 60 countries worldwide have approved the drug but it remains unapproved in North America, where it is currently in Phase III development by Tenax Therapeutics for reduction in morbidity and mortality of cardiac surgery patients at risk of low cardiac output syndrome.[6]
Contraindications
The use of levosimendan is contraindicated in patients with moderate-to-severe kidney impairment, severe liver impairment, severe ventricular filling or outflow obstruction, very low blood pressure and fast heart rate, and/or history of the abnormal heart rhythm torsades de pointes (Rossi, 2006).[7]
Adverse effects
Common adverse drug reactions (≥1% of patients) associated with levosimendan therapy include: headache, hypotension, arrhythmias (atrial fibrillation, extrasystoles, atrial tachycardia, ventricular tachycardia), myocardial ischaemia, hypokalaemia and/or nausea (Rossi, 2006).
Formulations
Levosimendan is marketed as a 2.5 mg/mL concentrated solution for IV infusion. The concentrate is diluted with glucose 5% solution before infusion.
References
- ↑ Papp, Z; Édes, I; Fruhwald, S; De Hert, SG; Salmenperä, M; Leppikangas, H; Mebazaa, A; Landoni, G; Grossini, E; Caimmi, P; Morelli, A; Guarracino, F; Schwinger, RH; Meyer, S; Algotsson, L; Wikström, BG; Jörgensen, K; Filippatos, G; Parissis, JT; González, MJ; Parkhomenko, A; Yilmaz, MB; Kivikko, M; Pollesello, P; Follath, F (23 August 2012). "Levosimendan: Molecular Mechanisms and Clinical Implications: Consensus of Experts on the Mechanisms of Action of Levosimendan". International Journal of Cardiology. 159 (2): 82–7. doi:10.1016/j.ijcard.2011.07.022. PMID 21784540.
- ↑ Nieminen, MS; Fruhwald, S; Heunks, LM; Suominen, PK; Gordon, AC; Kivikko, M; Pollesello, P (2013). "Levosimendan: Current Data, Clinical Use and Future Development". Heart, Lung and Vessels. 5 (4): 227–45. PMC 3868185
 . PMID 24364017.
. PMID 24364017. - 1 2 Mebazaa, A; Nieminen, MS; Packer, M; Cohen-Solal, A; Kleber, FX; Pocock, SJ; Thakkar, R; Padley, RJ; Põder, P; Kivikko, M; SURVIVE, Investigators (2 May 2007). "Levosimendan vs Dobutamine for Patients with Acute Decompensated Heart Failure: the SURVIVE Randomized Trial". JAMA. 297 (17): 1883–91. doi:10.1001/jama.297.17.1883. PMID 17473298.
- ↑ Landoni, G; Biondi-Zoccai, G; Greco, M; Greco, T; Bignami, E; Morelli, A; Guarracino, F; Zangrillo, A (February 2012). "Effects of Levosimendan on Mortality and Hospitalization. A Meta-analysis of Randomized Controlled Studies". Critical Care Medicine. 40 (2): 634–46. doi:10.1097/ccm.0b013e318232962a. PMID 21963578.
- ↑ Orion. "Simdax (levosimendan) Fact Sheet" (PDF). Orion. Retrieved 16 February 2013.
- ↑ Tenax Theraputics. "Levosimendan - Tenax Theraputics". Tenax Theraputics. Retrieved 27 November 2016.
- ↑ Rossi S, editor. Australian Medicines Handbook 2006. Adelaide: Australian Medicines Handbook; 2006.