Laurolactam
 | |
| Names | |
|---|---|
| IUPAC name
azacyclotridecan-2-one | |
| Other names
Dodecalactam | |
| Identifiers | |
| 947-04-6 | |
| 3D model (Jmol) | Interactive image |
| ChemSpider | 13099 |
| ECHA InfoCard | 100.012.204 |
| PubChem | 13690 |
| |
| |
| Properties | |
| C12H23NO | |
| Molar mass | 197.32 g·mol−1 |
| Appearance | colourless solid |
| Melting point | 152.5 °C (306.5 °F; 425.6 K)[1] |
| Boiling point | 314.9±10 °C |
| 0.03 wt% | |
| Hazards | |
| Flash point | 192 °C |
| 320 to 330 °C | |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Laurolactam is an organic compound with the molecular formula (CH2)11C(O)NH. This colorless solid is classified as a lactam. Laurolactam is mainly used as a monomer in engineering plastics, such as nylon-12 and copolyamides.
Synthesis
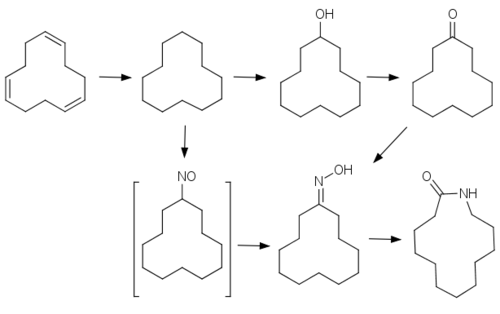
Although a derivative of 12-aminododecanoic acid, it is made from cyclododecatriene. The triene is hydrogenated to the saturated alkane, cyclododecane. In the process adopted by Evonik Industries, the cycloalkane is oxidized to the ketone cyclododecanone using a boric acid catalyst. The cyclic ketone is converted to the oxime by condensation with hydroxylamine. Under strong acidic conditions and elevated temperatures (115 °C), the oxime converts via a Beckmann rearrangement to laurolactam.
The procedure used by Arkema also begins cyclododecane, which is treated with nitrosyl chloride, resulting in the nitrosocyclododecane. This unstable species undergoes tautomeric transformation to the oxime, which is amenable to Beckmann rearrangement.[2]
Uses
Ring opening polymerization is used to polymerize the monomer to nylon-12. The reaction can be brought about with cationic or anionic initiators or with water . Cationic polymerization with acid is believed to involve the initial O-protonation. Nucleophilic attack by the monomer on the reactive protonated nitrogen, followed by successive ring-opening acylation of the primary amine results in the formation of the polyamide.[3]
References
- ↑ Bradley, Jean-Claude; Williams, Antony; Lang, Andrew (2014): Jean-Claude Bradley Open Melting Point Dataset. figshare. doi:10.6084/m9.figshare.1031637
- ↑ Schiffer, T.; Oenbrink, G. "Cyclododecanol, Cyclododecanone, and Laurolactam" in Ullman’s Encyclopedia of Industrial Chemistry: Wiley-VCH, 2009. doi:10.1002/14356007.a08_201.pub2
- ↑ Stevens, M. P. Polymer Chemistry : An Introduction, Oxford University Press: New York, 1999.