Labdane
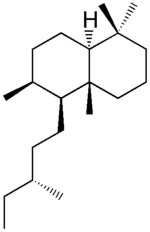 | |
| Names | |
|---|---|
| IUPAC name
(4aR,5S,6S,8aS)- 1,1,4a,6-tetramethyl-5- [(3R)-3-methylpentyl]decalin | |
| Identifiers | |
| 561-90-0 | |
| 3D model (Jmol) | Interactive image |
| ChEBI | CHEBI:36505 |
| ChEMBL | ChEMBL1087749 |
| ChemSpider | 7827634 |
| PubChem | 9548711 |
| |
| |
| Properties | |
| C20H38 | |
| Molar mass | 278.516 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Labdane is a natural bicyclic diterpene. It forms the structural core for a wide variety of natural products collectively known as labdanes or labdane diterpenes. The labdanes were so named because the first members of the class were originally obtained from labdanum, a resin derived from rockrose plants.[1][2]
A variety of biological activities have been determined for labdane diterpenes including antibacterial, antifungal, antiprotozoal, and anti-inflammatory activities.[3]
Examples
- Forskolin
- Stemodene
- Isocupressic acid, a labdane diterpenoid, is an abortifacient component of Cupressus macrocarpa.[4]
See also
References
- ↑ Cocker, J. D.; Halsall, T. G.; Bowers, A. (1956). "The chemistry of gum labdanum. I. Some acidic constituents". Journal of the Chemical Society: 4259–62.
- ↑ Cocker, J. D.; Halsall, T. G. (1956). "The chemistry of gum labdanum. II. The structure of labdanolic acid". Journal of the Chemical Society: 4262–71.
- ↑ Studies in Natural Product Chemistry : Bioactive Natural Products, Part F, Atta-Ur-Rahman (Editor), ISBN 978-0-08-044001-9
- ↑ Isocupressic acid, an abortifacient component of Cupressus macrocarpa. K. Parton, D. Gardner and N.B. Williamson, New Zealand Veterinary Journal, 1996, Volume 44, Issue 3, doi:10.1080/00480169.1996.35946
This article is issued from Wikipedia - version of the 6/1/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.