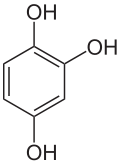
Hydroxyquinol
 | |
| Names | |
|---|---|
| IUPAC name
benzene-1,2,4-triol | |
| Other names
Hydroxyhydroquinone Oxyhydroquinone 1,2,4-Benzenetriol 1,2,4-Trihydroxybenzene Benzene-1,2,4-triol 4-Hydroxycatechol 2,5-Dihydroxyphenol 1,3,4-Benzenetriol 1,3,4-Trihydroxybenzene | |
| Identifiers | |
| 533-73-3 | |
| 3D model (Jmol) | Interactive image |
| ChEBI | CHEBI:16971 |
| ChemSpider | 10331 |
| ECHA InfoCard | 100.007.797 |
| KEGG | C02814 |
| PubChem | 10787 |
| |
| |
| Properties | |
| C6H6O3 | |
| Molar mass | 126.11 g/mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Hydroxyquinol is a benzenetriol.
Production
Hydroxyquinol is produced by various means of degradation. Historically hydroxyquinol was produced by the action of potassium hydroxide on hydroquinone.[1] Today the chemical is more conveniently synthesized by dehydrating fructose with supercritical water:[2][3]
- C6H12O6 → 3 H2O + C6H6O3
Natural Occurrence
Hydroxyquinol commonly occurs in nature as a biodegradation product of catechin, a natural phenol found in plants such as Bradyrhizobium japonicum.[4] Hydroxyquinol is also a metabolite in some organisms. For instance, Hydroxyquinol 1,2-dioxygenase is an enzyme that uses hydroxyquinol as a substrate with oxygen to produce 3-hydroxy-cis,cis-muconate.
References
- ↑ Roscoe, Henry (1891). A treatise on chemistry, Volume 3, Part 3. London: Macmillan & Co. p. 199.
- ↑ Luijkx, Gerard; Rantwijk, Fred; Bekkum, Herman (1993). "Hydrothermal formation of 1,2,4-benzenetriol from 5-hydroxymethyl-2-furaldehyde and D-fructose". Carbohydrate Research. 242 (1): 131–139. doi:10.1016/0008-6215(93)80027-C.
- ↑ Srokol, Zbigniew; Anne-Gaëlle, Bouche; Estrik, Anton; Strik, Rob; Maschmeyer, Thomas; Peters, Joop (2004). "Hydrothermal upgrading of biomass to biofuel; studies on some monosaccharide model compounds". Carbohydrate Research. 339 (10): 1717–1726. doi:10.1016/j.carres.2004.04.018.
- ↑ Mahadevan, A.; Waheeta, Hopper (1997). "Degradation of catechin by Bradyrhizobium japonicum". Biodegradation. 8 (3): 159–165. doi:10.1023/A:1008254812074.
This article is issued from Wikipedia - version of the 10/18/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.