Geraniol
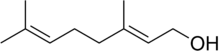 | |
 | |
| Names | |
|---|---|
| IUPAC name
(trans)-3,7-Dimethyl-2,6-octadien-1-ol | |
| Identifiers | |
| 106-24-1 | |
| 3D model (Jmol) | Interactive image |
| ChEBI | CHEBI:17447 |
| ChEMBL | ChEMBL25719 |
| ChemSpider | 13849989 |
| ECHA InfoCard | 100.003.071 |
| EC Number | 203-377-1 |
| 2467 | |
| PubChem | 637566 |
| UNII | L837108USY |
| |
| |
| Properties | |
| C10H18O | |
| Molar mass | 154.25 g·mol−1 |
| Density | 0.889 g/cm3 |
| Melting point | −15 °C (5 °F; 258 K)[2] |
| Boiling point | 230 °C (446 °F; 503 K)[2] |
| 686 mg/L (20 °C)[2] | |
| Hazards | |
| NFPA 704 | |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Geraniol is a monoterpenoid and an alcohol. It is the primary part of rose oil, palmarosa oil, and citronella oil (Java type). It also occurs in small quantities in geranium, lemon, and many other essential oils. It appears as a clear to pale-yellow oil that is insoluble in water, but soluble in most common organic solvents. It has a rose-like scent and is commonly used in perfumes. It is used in flavors such as peach, raspberry, grapefruit, red apple, plum, lime, orange, lemon, watermelon, pineapple, and blueberry.
Use
Research has shown geraniol to be an effective plant-based mosquito repellent.[3][4] On the other hand, it can attract bees as it is produced by the scent glands of honey bees to help them mark nectar-bearing flowers and locate the entrances to their hives.[5]
Although geraniol and other flavor compounds are found naturally in well-aged tobacco, geraniol is listed in a 1994 report from cigarette companies as one of the 599 additives to cigarettes to improve their flavor.[6]
Biochemistry
The functional group based on geraniol (in essence, geraniol lacking the terminal -OH) is called geranyl. It is important in biosynthesis of other terpenes. It is a by-product of the metabolism of sorbate and, thus, is a very unpleasant contaminant of wine if bacteria are allowed to grow in it.
Reactions
In acidic solutions, geraniol is converted to the cyclic terpene alpha-terpineol.
Health and safety
Geraniol is classified as D2B (Toxic materials causing other effects) using the Workplace Hazardous Materials Information System (WHMIS). Geraniol is considered a severe eye (and moderate skin) irritant.[7]
Related compounds
- Citral, the corresponding aldehyde
- Nerol, the double bond isomer
- Rhodinol, a related terpene alcohol
- Geranyl pyrophosphate
- Geranylgeranyl pyrophosphate
- Linalool, the isomer derived from transposition of the allylic alcohol
See also
References
- ↑ Geraniol, The Merck Index, 12th Edition
- 1 2 3 Record in the GESTIS Substance Database of the IFA
- ↑ Barnard, D.R.; Xue, R. (2004). "Laboratory evaluation of mosquito repellents against Aedes albopictus, Culex nigripalpus, and Ochlerotatus triseriatus (Diptera: Culicidae)". J. Med. Entomol. 41 (4): 726–730. doi:10.1603/0022-2585-41.4.726. PMID 15311467.
- ↑ UF entomologist develops safe, effective alternative to DEET insect repellents, Univ. of Florida, 1999
- ↑ R.G. Danka; J.L. Williams; T.E. Rinderer (1990). "A bait station for survey and detection of honey bees". Apidologie. 21 (4): 287–292. doi:10.1051/apido:19900403.
- ↑ What's in a cigarette? at about.com
- ↑ MSDS - Geraniol at www.sigmaaldrich.com, Accessed June 24, 2014
