GIR1 branching ribozyme
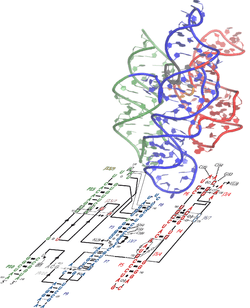
The Lariat capping ribozyme formerly called GIR1 branching ribozyme is a 179 nt ribozyme with an apparent resemblance to a group I ribozyme.[2] It is found within a complex type of group I introns also termed twin-ribozyme introns.[3] Rather than splicing, it catalyses a branching reaction in which the 2'OH of an internal residue is involved in a nucleophilic attack at a nearby phosphodiester bond.[4] As a result, the RNA is cleaved at an internal processing site (IPS), leaving a 3'OH and a downstream product with a tiny lariat at its 5' end. The lariat has the first and the third nucleotide joined by a 2',5' phosphodiester bond and is referred to as 'the lariat cap' because it caps an intron-encoded mRNA. The resulting lariat cap seems to contribute by increasing the half-life of the HE mRNA,[4][5] thus conferring an evolutionary advantage to the HE.
Biological context
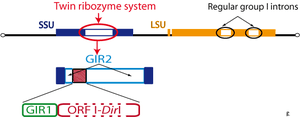
The GIR1 ribozyme was originally discovered during the functional characterization of the introns from the extrachromosomal rDNA of the Didymium iridis protist. A combination of deletion and in vitro self-splicing analyses revealed a twin-ribozyme intron organization: two distinct ribozyme domains within the intron.[3]
Structural organization of the twin-ribozyme Introns
The twin-ribozyme introns represent some of the most complex organized group I introns known and consist of a homing endonuclease gene (HEG: I-DirI homing endonuclease) embedded in two functionally distinct catalytic RNA domains. One of the catalytic RNAs is a conventional group I intron ribozyme (GIR2) responsible for the intron splicing and reverse splicing, as well as intron RNA circularization. The other catalytic RNA domain is the group I-like ribozyme (GIR1) directly involved in homing endonuclease mRNA maturation.
GIR1 catalyzes three different reactions
| GIR1 Branching Ribozyme | |
|---|---|
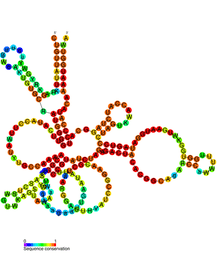 | |
| Conserved secondary structure of GIR1 | |
| Identifiers | |
| Symbol | GIR1 |
| Rfam | RF01807 |
| Other data | |
| RNA type | Intron |
| Domain(s) | Naegleria |
In vitro, DiGIR1 catalyses three different reactions. The first one consists in hydrolysis of the scissil phosphate at the IPS site. This is the cleavage reaction observed with the full-length intron and several length variants with a relative low rate. The hydrolytic cleavage is irreversible and is considered as an in vitro artefact resulting from a miss-folding of the catalytic site to present the branch nucleotide (BP) correctly for the reaction. The second reaction,the natural one, is the branching reaction, in which a transesterification at the IPS site results in the cleavage of the RNA with a 3'OH and a downstream lariat cap made by joining of the first and the third nucleotide by a 2'-5' phosphodiester bond.[4]
These products are the only products observed by analysis of cellular RNA.[5][6] This branching reaction is in equilibrium with a third one: a ligation reaction. It is a very efficient reaction and it tends to mask the branching reaction during the in vitro branching experiments with the full-length intron and length variants that include more than 166 nucleotides upstream of the IPS.
Modelling structure of GIR1
GIR1 models have been created using biochemical and mutational data.[1] The structure contains an extended substrate domain which contains a GoU pair. The pair differs from the typical group 1 ribozyme nucleophilic residue, the J8/7 region has been reduced.[1] These findings provide the basis for an evolutionary mechanism that accounts for the change from group I splicing ribozyme to the branching GIR1 architecture. This mechanism could potentially be applied to other large RNAs such as the ribonuclease P.[1]
References
- 1 2 3 4 Beckert, B.; Masquida B. (2008). "Molecular modelling of the GIR1 branching ribozyme gives new insight into evolution of structurally related ribozymes". The EMBO Journal. 27 (4): 667–78. doi:10.1038/emboj.2008.4. PMC 2219692
 . PMID 18219270.
. PMID 18219270. - ↑ Johansen, S.; Nielsen H. (September 2002). "DiGIR1 and NaGIR1: Naturally occurring group I-like ribozymes with unique core organization and evolved biological role". Biochimie. 84 (9): 905–12. doi:10.1016/S0300-9084(02)01443-8. PMID 12458083.
- 1 2 Johansen, S.; Vogt V.M. (February 1994). "An intron in the nuclear ribosomal DNA of Didymium iridis encodes for a group I ribozyme and a novel ribozyme that cooperate in self-plicing". Cell. 76 (4): 725–34. doi:10.1016/0092-8674(94)90511-8. PMID 8124711.
- 1 2 3 Nielsen, H.; Johansen S. (September 2005). "An mRNA is capped by a 2'5' lariat catalized by a group I-like ribozyme". Science. 309 (5740): 1584–7. doi:10.1126/science.1113645. PMID 16141078.
- 1 2 Vader, A.; Nielsen H. (December 2002). "The group I-like ribozyme DiGIR1 mediates alternative processing of pre-rRNA transcripts in Didymium iridis". Biochem. 269 (23): 5804–12. doi:10.1046/j.1432-1033.2002.03283.x. PMID 12444968.
- ↑ Vader, A.; Johansen S. (February 1999). "In vivo expression of the nucleolar group I intron-encoded I-DirI homing endonuclease involves the removal of a spliceosomal intron". EMBO J. 18 (4): 1003–13. doi:10.1093/emboj/18.4.1003. PMC 1171192
 . PMID 10022842.
. PMID 10022842.
Further reading
- Jäschke A (2008). "Book Review: Ribozymes and RNA Catalysis. Edited by David M. J. Lilley and Fritz Eckstein.". Angewandte Chemie International Edition. 47 (45): 8558–9. doi:10.1002/anie.200885598.
- Doherty EA, Doudna JA (2001). "Ribozyme structures and mechanisms". Annu Rev Biophys Biomol Struct. 30: 457–75. doi:10.1146/annurev.biophys.30.1.457. PMID 11441810.
- Visser CM (1984). "Evolution of Biocatalysis — Part One — Possible Pre-Genetic RNA Catalysts which are Their Own Replicas". Origins of Life. 14 (1-4): 291–300. doi:10.1007/BF00933670. PMID 6205343. RNA catalysis evolutionary insight
External links
- Labs working on GIR1 branching ribozyme characterisation:
- Henrik Nielsen lab
- Eric Westhof lab
- Steinar Johansen lab
- RNA catalysis
- Page for GIR1 branching ribozyme at Rfam