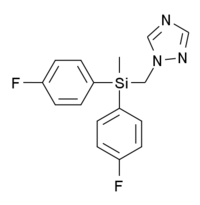
Flusilazole
 | |
 | |
| Names | |
|---|---|
| IUPAC name
1-((bis(4-fluorophenyl)methylsilyl)methyl)-1H-1,2,4-triazole | |
| Other names
DPX-H6573; | |
| Identifiers | |
| 85509-19-9 | |
| 3D model (Jmol) | Interactive image |
| ChEBI | CHEBI:81922 |
| ChemSpider | 66326 |
| ECHA InfoCard | 100.107.525 |
| PubChem | 73675 |
| |
| |
| Properties | |
| C16H15F2N3Si | |
| Density | 315.392 g/cm3 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Flusilazole (DPX-H6573) is an organosilicon fungicide invented by DuPont, which is used to control fungal infections on a variety of fruit and vegetable crops.[1][2][3] It is moderately toxic to animals and has been shown to produce birth defects and embryotoxicity at high doses.[4][5]
External links
- Flusilazole in the Pesticide Properties DataBase (PPDB)
References
- ↑ Moberg, W. K.; Basarab, G. S.; Cuomo, J.; Liang, P. H. (1987). "Biologically Active Organosilicon Compounds". 355: 288. doi:10.1021/bk-1987-0355.ch026.
- ↑ Bostanian NJ, Larocque N, Chouinard G, Coderre D (November 2001). "Baseline toxicity of several pesticides to Hyaliodes vitripennis (Say) (Hemiptera: Miridae)". Pest Management Science. 57 (11): 1007–10. doi:10.1002/ps.374. PMID 11721516.
- ↑ Eckert MR, Rossall S, Selley A, Fitt BD (April 2010). "Effects of fungicides on in vitro spore germination and mycelial growth of the phytopathogens Leptosphaeria maculans and L. biglobosa (phoma stem canker of oilseed rape)". Pest Management Science. 66 (4): 396–405. doi:10.1002/ps.1890. PMID 20013877.
- ↑ Farag AT, Ibrahim HH (February 2007). "Developmental toxic effects of antifungal flusilazole administered by gavage to mice". Birth Defects Research. Part B, Developmental and Reproductive Toxicology. 80 (1): 12–7. doi:10.1002/bdrb.20098. PMID 17187383.
- ↑ Hermsen SA, van den Brandhof EJ, van der Ven LT, Piersma AH (January 2011). "Relative embryotoxicity of two classes of chemicals in a modified zebrafish embryotoxicity test and comparison with their in vivo potencies". Toxicology in Vitro. 25 (3): 745–753. doi:10.1016/j.tiv.2011.01.005. PMID 21238576.
This article is issued from Wikipedia - version of the 7/2/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.