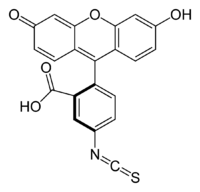
Fluorescein isothiocyanate
 | |
| Names | |
|---|---|
| Other names
FITC | |
| Identifiers | |
| 3326-32-7 | |
| 3D model (Jmol) | Interactive image |
| ChEBI | CHEBI:37928 |
| ChemSpider | 2043359 |
| ECHA InfoCard | 100.043.810 |
| MeSH | Fluorescein-5-isothiocyanate |
| PubChem | 18730 |
| UNII | 7BUU93N0HM |
| |
| |
| Properties | |
| C21H11NO5S | |
| Molar mass | 389.382 |
| Density | 1.542 g/mL |
| Melting point | 359.5 °C (679.1 °F; 632.6 K) |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Fluorescein isothiocyanate (FITC) is a derivative of fluorescein used in wide-ranging applications including flow cytometry. FITC is the original fluorescein molecule functionalized with an isothiocyanate reactive group (-N=C=S), replacing a hydrogen atom on the bottom ring of the structure. This derivative is reactive towards nucleophiles including amine and sulfhydryl groups on proteins.
A succinimidyl-ester functional group attached to the fluorescein core, creating "NHS-fluorescein", forms another common amine reactive derivative that has much greater specificity toward primary amines in the presence of other nucleophiles.
FITC has excitation and emission spectrum peak wavelengths of approximately 495 nm/519 nm.[1] Like most fluorochromes, it is prone to photobleaching. Because of the problem with photobleaching, derivatives of fluorescein such as Alexa 488 and DyLight 488 have been tailored for various chemical and biological applications where greater photostability, higher fluorescence intensity, or different attachment groups are needed.
References
- ↑ http://www.fluorophores.tugraz.at/substance/252 Fluorophores.org