Electron capture detector
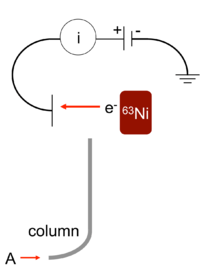
An electron capture detector (ECD) is a device for detecting atoms and molecules in a gas through the attachment of electrons via electron capture ionization. The device was invented in 1957 by James Lovelock[1][2][3] and is used in gas chromatography to detect trace amounts of chemical compounds in a sample.[4][5][6]
Gas chromatograph detector
.jpg)

The electron capture detector is used for detecting electron-absorbing components (high electronegativity) such as halogenated compounds in the output stream of a gas chromatograph. The ECD uses a radioactive beta particle (electron) emitter in conjunction with a so-called makeup gas flowing through the detector chamber. The electron emitter typically consists of a metal foil holding 10 millicuries (370 MBq) of the radionuclide 63
Ni
. Usually, nitrogen is used as makeup gas, because it exhibits a low excitation energy, so it is easy to remove an electron from a nitrogen molecule. The electrons emitted from the electron emitter collide with the molecules of the makeup gas, resulting in many more free electrons. The electrons are accelerated towards a positively charged anode, generating a current. There is therefore always a background signal present in the chromatogram. As the sample is carried into the detector by the carrier gas, electron-absorbing analyte molecules capture electrons and thereby reduce the current between the collector anode and a cathode. The analyte concentration is thus proportional to the degree of electron capture. ECD detectors are particularly sensitive to halogens, organometallic compounds, nitriles, or nitro compounds.
Sensitivity
Depending on the analyte, an ECD can be 10-1000 times more sensitive than a flame ionization detector (FID), and one million times more sensitive than a thermal conductivity detector (TCD). An ECD has a limited dynamic range and finds its greatest application in analysis of halogenated compounds.[7] The detection limit for electron capture detectors is 5 femtograms per second (fg/s), and the detector commonly exhibits a 10,000-fold linear range. This made it possible to detect halogenated compounds such as pesticides and CFCs, even at levels of only one part per trillion (ppt), thus revolutionizing our understanding of the atmosphere and pollutants.
References
- ↑ "Library and Archive Catalogue EC/1974/16: Lovelock, James Ephraim". London: The Royal Society. Archived from the original on 2014-04-10.
- ↑ Lovelock, J. E. (1958). "A sensitive detector for gas chromatography". Journal of Chromatography A. 1: 35. doi:10.1016/S0021-9673(00)93398-3.
- ↑ Lovelock, J. E. (1974). "The electron capture detector". Journal of Chromatography A. 99: 3. doi:10.1016/S0021-9673(00)90840-9.
- ↑ Krejči, M.; Dressler, M. (1970). "Selective detectors in gas chromatography". Chromatographic Reviews. 13: 1. doi:10.1016/0009-5907(70)80005-9.
- ↑ Pellizzari, E. D. (1974). "Electron capture detection in gas chromatography". Journal of Chromatography A. 98 (2): 323. doi:10.1016/S0021-9673(00)92077-6.
- ↑ Lovelock, J. E.; Maggs, R. J.; Wade, R. J. (1973). "Halogenated Hydrocarbons in and over the Atlantic". Nature. 241 (5386): 194. doi:10.1038/241194a0.
- ↑ Various. "Advisory Opinion". Innovative Technology: Gas Chromatography Field Analysis. NEWMOA Technology Review Committee. Retrieved 2011-04-21.