Disulfite
 | |
_Ion_Ball_and_Stick.png) | |
| Names | |
|---|---|
| IUPAC name
disulfite [1] | |
| Other names
metabisulfite ion pyrosulfite | |
| Identifiers | |
| PubChem | 159940 |
| Properties | |
| S 2O2− 5 | |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
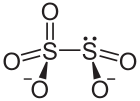
A disulfite, commonly known as metabisulfite or pyrosulfite, is a chemical compound containing the disulfite ion (metabisulfite ion) S
2O2−
5.
Structure
Surprisingly, and in contrast to disulfate (S
2O2−
7), disulfite ion (S
2O2−
5) has two directly connected sulfur atoms, and the structure is described as "thionite-thionate", or [O2S–SO3]2−, instead of the symmetrical form [O2S–O–SO2]2−.[2]
The oxidation state of the sulfur atom bonded to 3 oxygen atoms is +5 while oxidation number of other sulfur atom is +3.
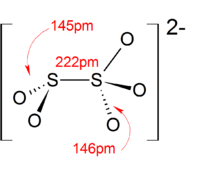
The anion consists of an SO2 group linked to an SO3 group, with the negative charge more localized on the SO3 end. The S–S bond length is 2.22 Å, and the "thionate" and "thionite" S–O distances are 1.46 and 1.50 Å respectively.[3]
Reactions
Production of the disulfite ion
The disulfite ion is a dimer of the bisulfite ion (HSO−
3). It can arise from:
In aqueous solution, the disulfite ion is formed in minor amounts by dehydration of bisulfite in an equilibrium:
- 2 HSO−
3(aq) S
S
2O2−
5(aq) + H2O (l)
Although the equilibrium lies far to the left, evaporation of a bisulfite salt will produce a substantial amount of disulfite.[4]
In fact, disulfite is the ion of disulfurous acid (pyrosulfurous acid), which originates from sulfurous acid in accordance with the dehydration reaction above:
- 2 H2SO3 → 2 HSO−
3 + 2 H+ → H2S2O5 + H2O
Addition
The disulfite ion also arises from the addition of sulfur dioxide to the sulfite ion:
| HSO− 3 3 + H+ SO32− + SO2 2O2− 5 | 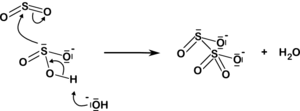 |
Other reactions
In aqueous solution, disulfite salts decompose with acids:
- S
2O2−
5 + H+ → HSO−
3 + SO2
Examples of disulfites
- Sodium metabisulfite (E223) and potassium metabisulfite (E224) are used as a preservative and antioxidant in food.
References
- ↑ International Union of Pure and Applied Chemistry (2005). Nomenclature of Inorganic Chemistry (IUPAC Recommendations 2005). Cambridge (UK): RSC–IUPAC. ISBN 0-85404-438-8. p. 130. Electronic version.
- ↑ I. Lindqvist and M. Mörtsell; "The structure of potassium pyrosulfite and the nature of the pyrosulfite ion". Acta Cryst. (1957). 10, 406–409. doi:10.1107/S0365110X57001322
- ↑ K. L. Carter, T. A. Siddiquee, K. L. Murphy, D. W. Bennett "The surprisingly elusive crystal structure of sodium metabisulfite" Acta Crystallogr. (2004). B60, 155–162. doi:10.1107/S0108768104003325
- ↑ Bassam Z. Shakhashiri: Chemical demonstrations: a handbook for teachers of chemistry The University of Wisconsin Press, 1992, p.9