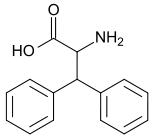
Diphenylalanine
 | |
| Names | |
|---|---|
| IUPAC name
2-amino-3,3-diphenyl-propionic acid | |
| Identifiers | |
| 149597-92-2 (L-isomer) 149597-91-1 (D-isomer) | |
| 3D model (Jmol) | Interactive image Interactive image |
| ChemSpider | 10608219 |
| ECHA InfoCard | 100.153.616 |
| |
| |
| Properties | |
| C15H15NO2 | |
| Molar mass | 241.11 g/mol |
| Appearance | Solid |
| Melting point | 235 °C (455 °F; 508 K) |
| Related compounds | |
| Related amino acids |
Alanine |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Diphenylalanine is an unnatural amino acid. It is similar to the two amino acids alanine and phenylalanine. It has been used for the synthesis of pseudopeptide analogues which are capable of inhibiting certain enzymes.[1]
A possible synthesis starts from 3,3-diphenyl-propionic acid which is stereoselective aminated to the diphenylalanine.[2]
References
- ↑ Leifeng Cheng; Christopher A. Goodwin; Michael F. Schully; Vijay V. Kakkar; Goran Claeson (1992). "Synthesis and biological activity of ketomethylene pseudopeptide analogs as thrombin inhibitors". Journal of Medicinal Chemistry. 35 (18): 3364–3369. doi:10.1021/jm00096a010. PMID 1527787.
- ↑ Huai G. Chen, V. G. Beylin, M. Marlatt, B. Leja and O. P. Goel, (1992). "Chiral cynthesis of D- and L-3,3-diphenylalanine (DIP), unusual α-amino acids for peptides of biological interest". Tetrahedron Letters. 33 (23): 3293–3296. doi:10.1016/S0040-4039(00)92070-7.
This article is issued from Wikipedia - version of the 11/22/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.