Dimethylphenylphosphine
 | |||
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Dimethylphenylphosphane | |||
| Other names
Dimethylphenylphosphine | |||
| Identifiers | |||
| 672-66-2 | |||
| 3D model (Jmol) | Interactive image | ||
| ChEBI | CHEBI:30671 | ||
| ChemSpider | 62800 | ||
| ECHA InfoCard | 100.010.543 | ||
| EC Number | 211-595-3 | ||
| PubChem | 69597 | ||
| |||
| |||
| Properties | |||
| C8H11P | |||
| Molar mass | 138.14 g/mol | ||
| Appearance | transparent light pale yellow liquid | ||
| Density | 0.971 g/cm3 | ||
| Melting point | N/A | ||
| Boiling point | 74 to 75 °C (165 to 167 °F; 347 to 348 K) at 12 mmHg | ||
| Insoluble | |||
| Structure | |||
| Pyramidal | |||
| Hazards | |||
| Safety data sheet | |||
| R-phrases | R10 R36 R37 R38 | ||
| S-phrases | S26 S36 | ||
| Flash point | 49 °C (120 °F; 322 K) | ||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| | |||
| Infobox references | |||
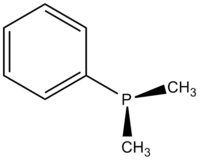
Dimethylphenylphosphine is an organophosphorus compound with a formula P(C6H5)(CH3)2. The phosphorus is connected to a phenyl group and two methyl groups, making it the simplest aromatic alkylphosphine. This colorless air sensitive liquid is commonly used as a ligand in transition metal complexes. These complexes are often soluble in organic solvents.
Preparation
Dimethylphenylphosphine is prepared by the reaction of methylmagnesium halide with dichlorophenylphosphine.
- (C6H5)Cl2P + 2CH3MgBr → (C6H5)(CH3)2P + 2MgBrCl
The phosphine is purified by distillation under reduced pressure.[1] A solution of (C6H5)(CH3)2P in CDCl3 shows proton NMR signals at δ 7.0-7.5 and a doublet at δ 1.2. The phosphorus-31 NMR spectrum shows a singlet at -45.89 ppm in CDCl3.[2]
Structure and properties
Dimethylphenylphosphine is a pyramidal molecule where the phenyl group and two methyl groups are connected to the phosphorus. The bond length and angles are the following: P-CMe: 1.844, P-CPh: 1.845 Å, C-C: 1.401 Å, C-HMe: 1.090 Å, C-HPh: 1.067 Å, C-P-C: 96.9°, C-P-C (ring): 103.4°, P-C-H: 115.2°.[3]
When attached to chiral metal centers, the P-methyl groups are diastereotopic, appearing as separate doublets in the 1H NMR spectrum.
Comparisons with related phosphine ligands
The nνCO of IrCl(CO)(PPh3)2 and IrCl(CO)(PMe2Ph)2 are both at 1960 cm−1, whereas nCO for Ir-Cl(CO)(PMe3)2 is at 1938 cm−1.[4][5]
The acidity of various protonated phosphine ligands gives insights into their basicity as ligands:[6][7]
- [HPMe2Ph]+= 6.8
- [HPPh3]+ = 2.7
- [HPEt3]+ = 8.7.
Thus PMe2Ph is intermediate in basicity relative to PPh3 and PEt3.
The ligand cone angle (θ) is the apex angle of a cylindrical cone, which is centered 2.28 Å from the center of the P atom. However, the cone angle of an unsymmetrical ligand cannot be determined in the same. In order to determine an effective cone angle for an unsymmetrical ligand PX1X2X3, the following equation is used:
Where θi represent the half angle.
The resulting angles for -PMe3, PMe2Ph, PPh3 are the following: PMe3= 118°, PMe2Ph= 122°, PPh3= 145°. Thus PMe2Ph is intermediate in size relative to PMe3 and PPh3.[8]
References
- ↑ C. Frajerman; B. Meunier; M. E. Strem., Inorganic Syntheses, Preparation of Dimethylphenylphosphine, 2007, volume 22, 133-135, doi:10.1002/9780470132531. ch29
- ↑ Laszlo T. Mika, Laszlo Orha, Norbert Farkas and Istvan T. Horvath, Organometallics, 2009, vol. 28, 1593
- ↑ Novikov, V. P.; Kolomeets, V. I., Syshchikov, Yu. N.; Vilkov, L. V.; Yarkov, A. V.; Tsvetkov, E. N.; Raevskii, O.A. "Investigation of structure of dimethylphenylphosphine by means of gas-phase electron diffraction and vibrational spectroscopy" Zh. Strukt. Khim. (J. Struc. Chem.) 1984, volume 25, No. 5, 688. doi:10.1007/BF00747909
- ↑ S.A. Cotton, Chemistry of Precious Metals., 1997, 152-157, ISBN 0-7514-0413-6, ISBN 978-0-7514-0413-5
- ↑ A. R. Norrris ; J. A. V. Kessel, Oxidative addition of 3,5-Dinitrobenzoyl Chloride to trans-Chlorocarbonylbis(dimethylphenylphosphine)iridium(I) Canadian Journal of Chemistry, 1973, volume 51, 4145-4151, doi:10.1139/CJC-51-24-4145.
- ↑ Russell C; Bush and Robert J. Angelici. Inorg. Chem, Phosphine Basicities As Determined by Enthalpies of Protonation 1988, 27, 681-686. doi:10.1021/ic00277a022
- ↑ Tianshu Li ; Alan J.; Robert H., Chemistry- A European Journal, An Acidity scale of Tetrafluoroborate Salts of Phosphonium and Iron Hydride compounds in [D2]Dichloromethane, 2007, volume 13, 3796-3803, doi: 10.1002/chem..200601484.
- ↑ C.A.Tolman, Chem.Rev., Steric effects of phosphorus ligands in organometallic chemistry and homogeneous catalysis., 1977, 313-348, doi:10.1021/cr60307a002