Dialysis (biochemistry)
In biochemistry, dialysis is the process of separating molecules in solution by the difference in their rates of diffusion through a semipermeable membrane, such as dialysis tubing.[1]
Dialysis is a common laboratory technique that operates on the same principle as medical dialysis. In the context of life science research, the most common application of dialysis is for the removal of unwanted small molecules such as salts, reducing agents, or dyes from larger macromolecules such as proteins, DNA, or polysaccharides.[2] Dialysis is also commonly used for buffer exchange and drug binding studies.
Principles of Dialysis
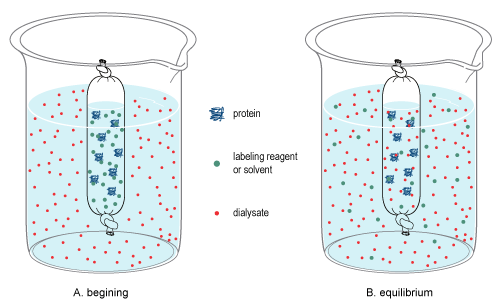
Diffusion is the random, thermal movement of molecules in solution (Brownian motion) that leads to the net movement of molecules from an area of higher concentration to a lower concentration until equilibrium is reached. In dialysis, a sample and a buffer solution (called the dialysate) are separated by a semi-permeable membrane that causes differential diffusion patterns, thereby permitting the separation of molecules in both the sample and dialysate.
Due to the pore size of the membrane, large molecules in the sample cannot pass through the membrane, thereby restricting their diffusion from the sample chamber. By contrast, small molecules will freely diffuse across the membrane and obtain equilibrium across the entire solution volume, thereby changing the overall concentration of these molecules in the sample and dialysate (see dialysis figure at right). Once equilibrium is reached, the final concentration of molecules is dependent on the volumes of the solutions involved, and if the equilibrated dialysate is replaced (or exchanged) with fresh dialysate (see procedure below), diffusion will further reduce the concentration of the small molecules in the sample.
Dialysis can be used to either introduce or remove small molecules from a sample, because small molecules move freely across the membrane in both directions. This makes dialysis a useful technique for a variety of applications. See dialysis tubing for additional information on the history, properties, and manufacturing of semi-permeable membranes used for dialysis.
Dialysis Procedure
Equipment
Separating molecules in a solution by dialysis is a straightforward process. Other than the sample and dialysate buffer, all that is typically needed is:
- Dialysis membrane in an appropriate format (e.g., tubing, cassette, etc.) and molecular weight cut-off (MWCO)
- A container to hold the dialysate buffer
- The ability to stir the solutions and control the temperature (optional)
General Protocol
A typical dialysis procedure for protein samples is as follows:
- Prepare the membrane according to instructions
- Load the sample into dialysis tubing, cassette or device
- Place sample into an external chamber of dialysis buffer (with gentle stirring of the buffer)
- Dialyze for 2 hours (at room temperature or 4 °C)
- Change the dialysis buffer and dialyze for another 2 hours
- Change the dialysis buffer and dialyze for 2 hours or overnight
The total volume of sample and dialysate determine the final equilibrium concentration of the small molecules on both sides of the membrane. By using the appropriate volume of dialysate and multiple exchanges of the buffer, the concentration of small contaminants within the sample can be decreased to acceptable or negligible levels. For example, when dialyzing 1mL of sample against 200mL of dialysate, the concentration of unwanted dialyzable substances will be decreased 200-fold when equilibrium is attained. Following two additional buffer changes of 200mL each, the contaminant level in the sample will be reduced by a factor of 8 x 106 (200 x 200 x 200).
Variables and Protocol Optimization
Although dialyzing a sample is relatively simple, a universal dialysis procedure for all applications cannot be provided due to the following variables:
- The sample volume
- The size of the molecules being separated
- The membrane used
- The geometry of the membrane, which affects the diffusion distance
Additionally, the dialysis endpoint is somewhat subjective and application specific. Therefore, the general procedure might require optimization.
Dialysis Membranes and MWCO
Dialysis membranes are produced and characterized according to molecular-weight cutoff (MWCO) limits. While membranes with MWCOs ranging from 1-1,000,000 kDa are commercially available, membranes with MWCOs near 10 kDa are most commonly used. The MWCO of a membrane is the result of the number and average size of the pores created during production of the dialysis membrane. The MWCO typically refers to the smallest average molecular mass of a standard molecule that will not effectively diffuse across the membrane during extended dialysis. Thus, a dialysis membrane with a 10K MWCO will generally retain greater than 90% of a protein having a molecular mass of at least 10kDa.[3][4]
It is important to note that the MWCO of a membrane is not a sharply defined value. Molecules with mass near the MWCO limit of the membrane will diffuse across the membrane more slowly than molecules significantly smaller than the MWCO. In order for a molecule to rapidly diffuse across a membrane, it typically needs to be at least 20- to 50-times smaller than the MWCO rating of a membrane. Therefore, it is not practical to separate a 30kDa protein from a 10kDa protein using dialysis across a 20K rated dialysis membrane.
Dialysis membranes for laboratory use are typically made of a film of regenerated cellulose or cellulose esters. See reference for a review of cellulose membranes and manufacturing.[5]
Laboratory Dialysis Formats
Dialysis is generally performed in clipped bags of dialysis tubing or in a variety of formatted dialyzers. The choice of the dialysis set up used is largely dependent on the size of the sample and the preference of the user. Dialysis tubing is the oldest and generally the least expensive format used for dialysis in the lab. Tubing is cut and sealed with a clip at one end, then filled and sealed with a clip on the other end. Tubing provides flexibility but has increased concerns regarding handling, sealing and sample recovery. Dialysis tubing is typically supplied either wet or dry in rolls or pleated telescoped tubes.
A wide variety of dialysis devices (or dialyzers) are available from several vendors. Dialyzers are designed for specific sample volume ranges and provide greater sample security and improved ease of use and performance for dialysis experiments over tubing. The most common preformatted dialyzers are Slide-A-Lyzer, Float-A-Lyzer, and the Pur-A-lyzer/D-Tube/GeBAflex Dialyzers product lines.
Suppliers
- Thermo Scientific
- Spectrum Laboratories
- Fisher Scientific
- EMD Millipore
- Sigma-Aldrich
- Harvard Apparatus
- Membrane Filtration Products, Inc.
References
- ↑ Reed, R (2007). Practical Skills in Biomolecular Sciences, 3rd ed. Essex: Pearson Education Limited. p. 379. ISBN 978-0-13-239115-3.
- ↑ Berg, JM (2007). Biochemistry, 6th ed. New York: W.H. Freeman and Company. p. 69. ISBN 0-7167-8724-5.
- ↑ "Separation characteristics of dialysis membranes". Retrieved 13 November 2013.
- ↑ "Fundamentals of membrane dialysis". Retrieved 13 November 2013.
- ↑ Klemm, Dieter; Brigitte Heublein; Hans-Peter Fink; Andreas Bohn (2005). "Cellulose: Fascinating Biopolymer and Sustainable Raw Material". Angewandte Chemie International Edition. 44 (22): 3358–3393. doi:10.1002/anie.200460587. PMID 15861454.