Bropirimine
 | |
| Clinical data | |
|---|---|
| ATC code | none |
| Identifiers | |
| |
| CAS Number | 56741-95-8 |
| ChemSpider | 58914 |
| UNII |
J57CTF25XJ |
| ChEMBL | CHEMBL37387 |
| ECHA InfoCard | 100.231.001 |
| Chemical and physical data | |
| Formula | C10H8BrN3O |
| Molar mass | 266.09 g/mol |
| 3D model (Jmol) | Interactive image |
| |
| |
Bropirimine is an experimental drug with anti-cancer and antiviral properties.[1] It is an orally effective immunomodulator and is being tried in bladder cancers.[2]
Synthesis
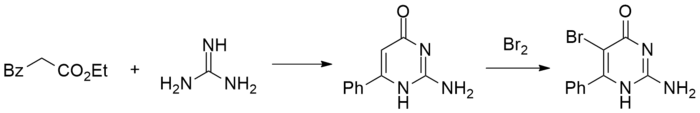
For the first step, the dianion from malonic acid half-ester is formed by treatment with butyllithium. Acylation of the anion with benzoyl chloride proceeds at the carbanion, which is more nucleophilic (because of the higher charge density). This tricarbonyl compound decarboxylates on acidification to the β-ketoester. Condensation with guanidine leads to the pyrimidone. NBS mediated bromination then gives bropirimine.
References
- ↑ sciencedirect
- ↑ http://content.karger.com/ProdukteDB/produkte.asp?Doi=19693
- ↑ Skulnick, Harvey I.; Weed, Sheldon D.; Eidson, Emerson E.; Renis, Harold E.; Stringfellow, Dale A.; Wierenga, Wendell (1985). "Pyrimidinones. 1. 2-Amino-5-halo-6-aryl-4(3H)-pyrimidinones. Interferon-inducing antiviral agents". Journal of Medicinal Chemistry. 28 (12): 1864–9. doi:10.1021/jm00150a018. PMID 2999405.
- ↑ Brown, Thomas B.; Stevens, Malcolm F. G. (1975). "Triazines and related products. Part XV. 2,4-Diaminopyrimidines and 2-aminopyrimidin-4(3H)-ones bearing 1,2,3-benzotriazinyl groups as potential dihydrofolic reductase inhibitors". Journal of the Chemical Society, Perkin Transactions 1 (11): 1023. doi:10.1039/p19750001023. ISSN 0300-922X.
- ↑ M. F. G. Stevens et al., Anti-Cancer Drug Des. 10, 215 (1995).
This article is issued from Wikipedia - version of the 4/10/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.