Benzoin
 | |
 | |
| Names | |
|---|---|
| IUPAC name
2-hydroxy-1,2-di(phenyl)ethanone | |
| Other names
2-hydroxy-2-phenylacetophenone, 2-hydroxy-1,2-diphenylethanone, desyl alcohol, bitter almond oil camphor | |
| Identifiers | |
| 119-53-9 | |
| 3D model (Jmol) | Interactive image Interactive image |
| ChEBI | CHEBI:17682 |
| ChEMBL | ChEMBL190677 |
| ChemSpider | 8093 |
| ECHA InfoCard | 100.003.938 |
| KEGG | C01408 |
| PubChem | 8400 |
| UNII | L7J6A1NE81 |
| |
| |
| Properties | |
| C14H12O2 | |
| Molar mass | 212.25 g·mol−1 |
| Appearance | off-white crystals |
| Density | 1.31 g/cm3 |
| Melting point | 132 to 137 °C (270 to 279 °F; 405 to 410 K) |
| Boiling point | 344 °C (651 °F; 617 K) |
| Slightly Soluble | |
| Solubility in ethanol | Slightly Soluble |
| Solubility in alcohol | Soluble |
| Solubility in ether | Slightly Soluble |
| Solubility in chlorine | Soluble |
| Hazards | |
| R-phrases | R36/38 [1] |
| NFPA 704 | |
| Lethal dose or concentration (LD, LC): | |
| LD50 (median dose) |
10.000 mg/kg |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
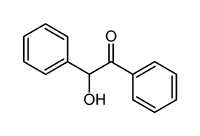
Benzoin (/ˈbɛnzoʊ.ᵻn/ or /-ɔɪn/) is an organic compound with the formula PhCH(OH)C(O)Ph. It is a hydroxy ketone attached to two phenyl groups. It appears as off-white crystals, with a light camphor-like odor. Benzoin is synthesized from benzaldehyde in the benzoin condensation. It is chiral and it exists as a pair of enantiomers: (R)-benzoin and (S)-benzoin.
Benzoin is not a constituent of benzoin resin obtained from the benzoin tree (Styrax) or tincture of benzoin. The main component in these natural products is benzoic acid.
History
Benzoin was first reported in 1832 by Justus von Liebig and Friedrich Woehler during their research on oil of bitter almond, which is benzaldehyde with traces of hydrocyanic acid.[2] The catalytic synthesis by the benzoin condensation was improved by Nikolay Zinin during his time with Liebig.[3][4]
Uses
The main uses of benzoin are as a precursor to benzil, which is a photoinitiator.[5] The conversion proceeds by organic oxidation using copper(II),[6] nitric acid, or oxone. In one study, this reaction is carried out with atmospheric oxygen and basic alumina in dichloromethane.[7]
Preparation
Benzoin is prepared from benzaldehyde via the benzoin condensation.[8]
References
- ↑ "Benzoin" (PDF). FischerSci. FischerSci.
- ↑ Wöhler, Liebig; Liebig (1832). "Untersuchungen über das Radikal der Benzoesäure". Annalen der Pharmacie. 3 (3): 249–282. doi:10.1002/jlac.18320030302.
- ↑ N. Zinin (1839). "Beiträge zur Kenntniss einiger Verbindungen aus der Benzoylreihe". Annalen der Pharmacie. 31 (3): 329–332. doi:10.1002/jlac.18390310312.
- ↑ N. Zinin (1840). "Ueber einige Zersetzungsprodukte des Bittermandelöls". Annalen der Pharmacie. 34 (2): 186–192. doi:10.1002/jlac.18400340205.
- ↑ Hardo Siegel, Manfred Eggersdorfer "Ketones" in Ullmann's Encyclopedia of Industrial Chemistry Wiley-VCH, 2002 by Wiley-VCH, Wienheim. doi:10.1002/14356007.a15_077
- ↑ Clarke, H. T.; Dreger.E. E. (1941). "Benzil". Org. Synth.; Coll. Vol., 1, p. 87
- ↑ Konstantinos Skobridis; Vassiliki Theodorou; Edwin Weber (2006). "A very simple and chemoselective air oxidation of benzoins to benzils using alumina". Arkivoc. 06-1798JP: 102–106.
- ↑ Clarke, H. T.; Dreger.E. E. (1941). "Benzil". Org. Synth.; Coll. Vol., 1, p. 87
External links
- Benzoin synthesis, Organic Syntheses, Coll. Vol. 1, p. 94 (1941); Vol. 1, p. 33 (1921)
