Astaxanthin
 | |
 | |
| Names | |
|---|---|
| IUPAC name
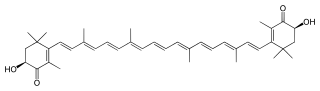
(6S)-6-Hydroxy-3-[(1E,3E,5E,7E,9E,11E,13E,15E,17E)-18-[(4S)-4-hydroxy-2,6,6-trimethyl-3-oxo-1-cyclohexenyl]-3,7,12,16-tetramethyloctadeca-1,3,5,7,9,11,13,15,17-nonaenyl]-2,4,4-trimethyl-1-cyclohex-2-enone | |
| Other names
3,3'-dihydroxy-ß-carotene-4,4'-dione; Astaxanthin (6CI); β-Carotene-4,4'-dione, 3,3'-dihydroxy-, all-trans- (8CI); (3S,3'S)-Astaxanthin; (3S,3'S)-Astaxanthin; (3S,3'S)-all-trans-Astaxanthin; (S,S)-Astaxanthin; Aquasta; AstaREAL; AstaXin; Astared; Astaxanthin, all-trans-; Astots 10O; Astots 5O; BioAstin; BioAstin oleoresin; Carophyll Pink; Lucantin Pink; NatuRose; Natupink; Ovoester; all-trans-Astaxanthin; trans-Astaxanthin [1] | |
| Identifiers | |
| 472-61-7 | |
| 3D model (Jmol) | Interactive image |
| ChEBI | CHEBI:40968 |
| ChEMBL | ChEMBL445751 |
| ChemSpider | 4444636 |
| ECHA InfoCard | 100.006.776 |
| E number | E161j (colours) |
| PubChem | 5281224 |
| UNII | 8XPW32PR7I |
| |
| |
| Properties | |
| C40H52O4 | |
| Molar mass | 596.84 g/mol |
| Appearance | red solid powder |
| Density | 1.071 g/mL [2] |
| Melting point | 216 °C (421 °F; 489 K)[2] |
| Boiling point | 774 °C (1,425 °F; 1,047 K)[2] |
| Solubility | 30 g/L in DCM; 10 g/L in CHCl3; 0.5 g/L in DMSO; 0.2 g/L in acetone [3] |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Astaxanthin /æstəˈzænθᵻn/ is a keto-carotenoid.[4][5] It belongs to a larger class of chemical compounds known as terpenes, which are built from five carbon precursors; isopentenyl diphosphate (or IPP) and dimethylallyl diphosphate (or DMAPP). Astaxanthin is classified as a xanthophyll (originally derived from a word meaning "yellow leaves" since yellow plant leaf pigments were the first recognized of the xanthophyll family of carotenoids), but currently employed to describe carotenoid compounds that have oxygen-containing moities, hydroxyl (-OH) or ketone (C=O), such as zeaxanthin and canthaxanthin. Indeed, astaxanthin is a metabolite of zeaxanthin and/or canthaxanthin, containing both hydroxyl and ketone functional groups. Like many carotenoids, astaxanthin is a colorful, lipid-soluble pigment. This colour is due to the extended chain of conjugated (alternating double and single) double bonds at the centre of the compound. This chain of conjugated double bonds is also responsible for the antioxidant function of astaxanthin (as well as other carotenoids) as it results in a region of decentralized electrons that can be donated to reduce a reactive oxidizing molecule.
Astaxanthin is found in microalgae, yeast, salmon, trout, krill, shrimp, crayfish, crustaceans, and the feathers of some birds. It provides the red color of salmon meat and the red color of cooked shellfish. Professor Basil Weedon's group was the first to prove the structure of astaxanthin by synthesis, in 1975.[6]
Astaxanthin, unlike several carotenes and one other known carotenoid, is not converted to vitamin A (retinol) in the human body. Like other carotenoids, astaxanthin has self-limited absorption orally and such low toxicity by mouth that no toxic syndrome is known. It is an antioxidant with a slightly lower antioxidant activity in some model systems than other carotenoids. However, in living organisms the free-radical terminating effectiveness of each carotenoid is heavily modified by its lipid solubility, and thus varies with the type of system being protected.[7]
While astaxanthin is a natural dietary component, it can also be used as a food supplement. The supplement is intended for human, animal, and aquaculture consumption. The industrial production of astaxanthin comes from both natural and synthetic sources.
The U.S. Food and Drug Administration (FDA) has approved astaxanthin as a food coloring (or color additive) for specific uses in animal and fish foods.[8] The European Commission considers it food dye and it is given the E number E161j.[9] Natural astaxanthin is generally recognized as safe (GRAS) by the FDA,[10][11] but as a food coloring in the United States it is restricted to use in animal food.[12]
Natural sources

.jpg)
Astaxanthin is present in most red-coloured aquatic organisms. The content varies from species to species, but also from individual to individual as it is highly dependent on diet and living conditions. Astaxanthin, and other chemically related asta-carotenoids, has also been found in a number of lichen species of the arctic zone.
The primary natural sources for commercial production of astaxanthin comprise the following:
- Euphausia pacifica (Pacific krill)
- Euphausia superba (Antarctic krill)
- Haematococcus pluvialis (MicroAlgae)[4]
- Pandalus borealis (Arctic shrimp)
- Xanthophyllomyces dendrorhous, formerly Phaffia rhodozyma (yeast)
Astaxanthin concentrations in natural sources as found in nature are approximately:
| Source | Astaxanthin concentration (ppm) |
|---|---|
| Salmonids | ~ 5 |
| Plankton | ~ 60 |
| Krill | ~ 120 |
| Arctic shrimp (P borealis) | ~ 1,200 |
| Phaffia yeast | ~ 10,000 |
| Haematococcus pluvialis | ~ 40,000 |
Algae are the primary natural source of astaxanthin in the aquatic food chain. Currently, the primary industrial source for natural astaxanthin is the microalgae Haematococcus pluvialis. Haematococcus pluvialis seems to accumulate the highest levels of astaxanthin in nature. Commercially, more than 40 g of astaxanthin can be obtained from one kg of dry biomass.[13] Haematococcus pluvialis has the advantage of the population doubling every week, which means scaling up is not an issue. However, it does require some expertise to grow the algae with a high astaxanthin content. Specifically, the microalgae are grown in two phases. First, in the green phase, the cells are given an abundance of nutrients to promote proliferation of the cells. In the subsequent red phase, the cells are deprived of nutrients and subjected to intense sunlight to induce encystment (carotogenesis), during which the cells produce high levels of astaxanthin as a protective mechanism against the environmental stress. The cells, with their high concentrations of astaxanthin, are then harvested.[14]
Phaffia yeast Xanthophyllomyces dendrorhous exhibits 100% free, non-esterified astaxanthin, which is considered advantageous because it is readily absorbable and need not be hydrolysed in the digestive tract of the fish. In contrast to synthetic and bacteria sources of astaxanthin, yeast sources of astaxanthin consist mainly of the (3R, 3’R)-form, an important astaxanthin source in nature. Finally, the geometrical isomer, all-E, is higher in yeast sources of astaxanthin, as compared to synthetic sources. This contributes to greater efficacy because the all-E (trans) isomer has greater bio-availability than the cis isomer.[15]
In shellfish, astaxanthin is almost exclusively concentrated in the shells, with only low amounts in the flesh itself, and most of it only becomes visible during cooking, as the pigment separates from the denatured proteins that otherwise binds it. For obtaining astaxanthin from Euphausia superba (Antarctic krill), there are a number of issues:[16]
The Krill fishing operation is complex. It is done in Antarctic waters, under extreme weather conditions and far away from ports with substantial operational complexities. Krill's fishing location and the difficult weather conditions in the main fishing area, together with the costs involved in the operation, have contributed to a slow development of the industry. Krill fishing is by far different than any other fishing operation today known. The knowledge to work with it belongs to very few people in the world.
Astaxanthin is also commercially collected from shrimp processing waste. 12,000 pounds of wet shrimp shells can yield a 6–8 gallon astaxanthin/triglyceride oil mixture.[17]
Synthetic sources
Nearly all commercial astaxanthin for aquaculture is produced synthetically, with an annual turnover of over $200 million and a selling price of roughly $5000–6000 per kilo as of July 2012.[13] However, synthetic production of astaxanthin is not preferred in some cases because synthetic astaxanthin contains a mixture of stereoisomers. Astaxanthin is fairly abundant and obtainable from natural sources, and some consumers prefer natural products over synthetic ones.[14]
An efficient synthesis from isophorone, cis-3-methyl-2-penten-4-yn-1-ol and a symmetrical C10-dialdehyde has been discovered and is used commercially. It combines these chemicals together with an ethynylation and then a Wittig reaction.[18] Two equivalents of the proper ylide combined with the proper dialdehyde in a solvent of methanol, ethanol, or a mixture of the two, yields astaxanthin in up to 88% yields.[19]

Metabolic engineering
The cost of astaxanthin production, high commercial price and lack of a leading fermentation production systems, combined with the shortfalls of chemical synthesis mean that research into alternative fermentation production methods has been carried out. Metabolic engineering offers the opportunity to create biological systems for the production of a specific target compound. The metabolic engineering of bacteria (Escherichia coli) recently allowed production of astaxanthin at >90% of the total carotenoids, providing the first engineered production system capable of efficient astaxanthin production.[20] Astaxanthin biosynthesis proceeds from beta-carotene via either zeaxanthin or canthaxanthin. Historically, it has been assumed that astaxanthin biosynthesis proceeds along both routes. However, recent work has suggested that efficient biosynthesis may, in fact, proceed from beta-carotene to astaxanthin via zeaxanthin.[21][22] The production of astaxanthin by metabolic engineering, in isolation, will not provide a suitable alternative to current commercial methods. Rather, a bioprocess approach should be adopted. Such an approach would consider fermentation conditions and economics, as well as downstream processing (extraction). Carotenoid extraction has been studied extensively, for example, the extraction of canthaxanthin (a precursor to astaxanthin) was studied within an E. coli production process demonstrating that extraction efficiency was increased substantially when two solvents, acetone and methanol, were used sequentially rather than as a combined solution.[23]
Difference between natural and synthetic forms
Astaxanthin has two chiral centers, at the 3- and 3′-positions. Therefore, there are three stereoisomers; (3R,3′R), (3R,3′S) (meso), and (3S,3′S). Synthetic astaxanthin contains a mixture of the three, in approximately 1:2:1 proportions. Naturally occurring astaxanthin varies considerably from one organism to another. The astaxanthin in fish is of whatever stereoisomer the fish ingested.[24] The astaxanthin produced by Haematococcus pluvialis, which is commonly used in the feed of animals that are in turn consumed by humans, is the (3S,3′S) stereoisomer.[14]
Uses
Astaxanthin is used as a feed supplement for salmon, crabs, shrimp, chickens and egg production.[25]
For seafood and animals
The primary use of synthetic astaxanthin today is as an animal feed additive to impart coloration, including farm-raised salmon and egg yolks.[13] Synthetic carotenoid pigments colored yellow, red or orange represent about 15–25% of the cost of production of commercial salmon feed.[26] Today, almost all commercial astaxanthin for aquaculture is produced synthetically from petrochemical sources.[27] While it constitutes a tiny portion of salmon feed (50 to 100 parts per million), astaxanthin represents a major share of the cost, up to 20%.[28]
Class action lawsuits have been filed against some major grocery store chains for not clearly labeling the salmon "color added".[28] The chains followed up quickly by labeling all such salmon as "color added". "...law-firm Smith & Lowney persisted with the suit for damages, but a Seattle judge dismissed [the case], ruling that enforcement of the applicable food laws was up to government and not individuals."[29]
For humans
The primary use for humans is as a dietary supplement. Research suggests that, due to astaxanthin's antioxidant activity, it may be beneficial in cardiovascular, immune, inflammatory and neurodegenerative diseases.[30] Some research supports the assumption that it may protect body tissues from oxidative and ultraviolet damage through its suppression of NF-κB activation.[31][32]
A 2015 meta-analysis of data from ten randomized, controlled trial groups in seven published clinical trials, doses ranging 4 to 20 mg/day, did not indicate a significant effect of supplementation with astaxanthin on plasma lipids profile or fasting glucose.[33]
Role in the food chain
It has been speculated that gulls are "flushed" pink when molting, especially in areas with farm-raised salmon.[34] However, not enough is known about the relationship between astaxanthin and plumage.[35] For example, cardinals seem to produce astaxanthin from carotenoids when molting, even when fed primarily seed with natural yellow dye.
Lobsters, shrimp, and some crabs turn red when cooked because the astaxanthin, which was bound to the protein in the shell, becomes free as the protein denatures and unwinds. The freed pigment is thus available to absorb light and produce the red color.[36]
Regulations
In April 2009, the United States Food and Drug Administration approved astaxanthin as an additive for fish feed only as a component of a stabilized color additive mixture. Color additive mixtures for fish feed made with astaxanthin may contain only those diluents that are suitable.[8] The color additives astaxanthin, ultramarine blue, canthaxanthin, synthetic iron oxide, dried algae meal, Tagetes meal and extract, and corn endosperm oil are approved for specific uses in animal foods.[37] Haematococcus algae meal (21 CFR 73.185) and Phaffia yeast (21 CFR 73.355) for use in fish feed to color salmonoids were added in 2000.[38][39][40] In the European Union, astaxanthin-containing food supplements derived from sources that have no history of use as a source of food in Europe, fall under the remit of the Novel Food legislation, EC (No.) 258/97. Since 1997, there have been five novel food applications concerning products that contain astaxanthin extracted from these novel sources. In each case, these applications have been simplified or substantial equivalence applications, because astaxanthin is recognised as a food component in the EU diet.[41][42][43][44]
References
- ↑ SciFinder Web (accessed Sep 28, 2010). Astaxanthin (472-61-7) Name
- 1 2 3 SciFinder Web (accessed Sep 28, 2010). Astaxanthin (472-61-7) Experimental Properties.
- ↑ Hussein G, Goto H, Oda S, Sankawa U, Matsumoto K, Watanabe H (April 2006). "Antihypertensive potential and mechanism of action of astaxanthin: III. Antioxidant and histopathological effects in spontaneously hypertensive rats". Biol. Pharm. Bull. 29 (4): 684–8. doi:10.1248/bpb.29.684. PMID 16595899.
- 1 2 Margalith, P. Z. (1999). "Production of ketocarotenoids by microalgae". Applied microbiology and biotechnology. 51 (4): 431–8. PMID 10341427.
- ↑ Choi, Seyoung; Koo, Sangho (2005). "Efficient Syntheses of the Keto-carotenoids Canthaxanthin, Astaxanthin, and Astacene". The Journal of Organic Chemistry. 70 (8): 3328. doi:10.1021/jo050101l. PMID 15823009.
- ↑ Cooper, R. D. G.; Davis, J. B.; Leftwick, A. P.; Price, C.; Weedon, B. (1975). "Carotenoids and related compounds. XXXII. Synthesis of astaxanthin, hoenicoxanthin, hydroxyechinenone, and the corresponding diosphenols". J. Chem. Soc. Perkin Trans. 1 (21): 2195–2204. doi:10.1039/p19750002195.
- ↑ Mortensen, A.; Skibsted, L. H. (1997). "Importance of carotenoid structure in radical scavenging reactions". J. Agric. Food Chem. 45 (8): 2970–7. doi:10.1021/jf970010s.
- 1 2 "Summary of Color Additives for Use in United States in Foods, Drugs, Cosmetics, and Medical Devices". See Note 1.
- ↑ E-numbers : E100- E200 Food Colours. Food-Info.net. Retrieved on 2013-04-25.
- ↑ Astaxanthin wins full GRAS status. Nutraingredients-usa.com. Retrieved on 2013-04-25.
- ↑ Algatechnologies gets GRAS for AstaPure astaxanthin. Foodnavigator-usa.com. Retrieved on 2013-04-25.
- ↑ Summary of Color Additives for Use in the United States in Foods, Drugs, Cosmetics, and Medical Devices. Fda.gov. Retrieved on 2013-04-25.
- 1 2 3 Astaxanthin – a superb natural antioxidant. algatech.com
- 1 2 3 Boussiba; Sammy, V.; Avigad, C.; et al. (2000) Procedure for large-scale production of astaxanthin from haematococcus. U. S. Patent 6,022,701.
- ↑ Natural vs. Synthetic Astaxanthin Sources. naturxan.com
- ↑ Katevas, Dimitri Sclabos (6 October 2003). The Krill. aquafeed.com
- ↑ Anderson, Lyle K. Extraction of Carotenoid Pigment from Shrimp Processing Waste. U.S. Patent 3906112. Sep 16, 1975
- ↑ Ashford's Dictionary of Industrial Chemicals, 3rd Edition, 2011, p. 984, ISBN 095226742X.
- ↑ Krause, Wolfgang; Henrich, Klaus; Paust, Joachim; et al. Preaparation of Astaxanthin. DE 19509955. 9 March 18, 1995
- ↑ Scaife, M. A.; Burja, A. M.; Wright, P. C. (2009). "Characterization of cyanobacterial β-carotene ketolase and hydroxylase genes inEscherichia coli, and their application for astaxanthin biosynthesis". Biotechnology and Bioengineering. 103 (5): 944–955. doi:10.1002/bit.22330. PMID 19365869.
- ↑ Scaife, MA; Ma, CA; Ninlayarn, T; Wright, PC; Armenta, RE (May 22, 2012). "Comparative Analysis of β-Carotene Hydroxylase Genes for Astaxanthin Biosynthesis". Journal of Natural Products. 75 (6): 1117–24. doi:10.1021/np300136t. PMID 22616944.
- ↑ Lemuth, K; Steuer, K; Albermann, C (Apr 26, 2011). "Engineering of a plasmid-free Escherichia coli strain for improved in vivo biosynthesis of astaxanthin". Microbial cell factories. 10: 29. doi:10.1186/1475-2859-10-29. PMC 3111352
 . PMID 21521516.
. PMID 21521516. - ↑ Scaife, M.A.; Ma, C.A.; Armenta, R.E. (May 2012). "Efficient extraction of canthaxanthin from Escherichia coli by a 2-step process with organic solvents". Bioresource Technology. 111: 276–281. doi:10.1016/j.biortech.2012.01.155. PMID 22353211.
- ↑ US application 20050014824 (online here) (also EP 1442083)
- ↑ Astaxanthin and Health and Wellness in Animals. astaxanthin.org
- ↑ Fisheries and Oceans Canada — Aquaculture Issues. pac.dfo-mpo.gc.ca.
- ↑ Juan F Martín, Eduardo Gudiña and José L Barredo (February 20, 2008). "Conversion of β-carotene into astaxanthin: Two separate enzymes or a bifunctional hydroxylase-ketolase protein?". Microbial Cell Factories. Microbial Cell Factories. Retrieved August 28, 2015.
- 1 2 "Smith & Lowney — Farm-raised Salmon Coloring". 2003. Retrieved 14 Oct 2009. (registration required)
- ↑ "Pigments in Salmon Aquaculture: How to Grow a Salmon-colored Salmon". Archived from the original on 13 Oct 2007. Retrieved 18 July 2009.
- ↑ Fassett, Robert G.; Coombes, Jeff S. (2009). "Astaxanthin, oxidative stress, inflammation and cardiovascular disease". Future Cardiology. 4 (3): 333–342. doi:10.2217/fca.09.19. PMID 19656058.
- ↑ Guerin M, Huntley ME, Olaizola M (2003). "Haematococcus astaxanthin: applications for human health and nutrition" (PDF). Trends Biotechnol. 21 (5): 210–6. doi:10.1016/S0167-7799(03)00078-7. PMID 12727382.
- ↑ Lennikov A; Nobuyoshi K; Risa Fukase (2012). "Amelioration of ultraviolet-induced photokeratitis in mice treated with astaxanthin eye drops" (PDF). Mollecular Vision. 18: 455–64. PMC 3291518
 .
. - ↑ Ursoniu S, Sahebkar A, Serban MC, Banach M (2015). "Lipid profile and glucose changes after supplementation with astaxanthin: a systematic review and meta-analysis of randomized controlled trials". Arch Med Sci. 11 (2): 253–66. doi:10.5114/aoms.2015.50960. PMC 4424245
 . PMID 25995739.
. PMID 25995739. - ↑ McGraw, Kevin; Hardy, Lisa (2006). "Astaxanthin is responsible for the pink plumage flush in Franklin's and Ring-billed gulls" (PDF). Tempe, AZ: School of Life Sciences, Arizona State University: 5.
- ↑ "Notes on the effects of Astaxanthin on the plumage of birds". didgood.com. 2006. Retrieved 19 July 2009.
- ↑ Why do crabs, lobsters & shrimp turn red when cooked?. Ochef.com. Retrieved on 2013-04-25.
- ↑ See 21 CFR 73.35,73.50, 73.75, 73.200, 73.275, 73.295, 73.315, respectively.
- ↑ Code of Federal Regulations Title 21 §73.35 FDA decision on Astaxanthin. Accessdata.fda.gov. Retrieved on 2013-04-25.
- ↑ Code of Federal Regulations Title 21 §73.185 FDA decision on Haematococcus algae meal. Accessdata.fda.gov. Retrieved on 2013-04-25.
- ↑ Food Additive Status List. fda.gov}
- ↑ Astaxanthin extract. acnfp.food.gov.uk
- ↑ Astaxanthin extract: Cyanotech Corporation. acnfp.gov.uk
- ↑ Astaxanthin extract: Algatechnologies (1998) Ltd. acnfp.gov.uk
- ↑ Astaxanthin extract: Parry Nutraceuticals. acnfp.gov.uk
External links
- Development of microalgal pigments for aquaculture in Europe; Final Report, February, 2001.
- Study of the expression of carotenoid biosynthesis genes in wild-type and deregulated strains of Xanthophyllomyces dendrorhous (Ex.: Phaffia rhodozyma).
- Multibudding in Xanthophyllomyces dendrorhous Cells Under Hydric and Nitrogen Stress
- The Effects of Three Carotenoid Sources on Growth and Pigmentation of Juvenile Freshwater Crayfish Cherax quadricarinatus