Acryloyl group
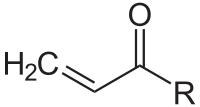
Structure of the acryloyl group
In organic chemistry, the acryloyl group is form of enone with structure H2C=CH–C(=O)–; it is the acyl group derived from acrylic acid. The preferred IUPAC name for the group is prop-2-enoyl, and it is also (less correctly) known as acrylyl or simply acryl. Compounds containing an acryloyl group can be referred to as "acrylic compounds".
An acrylic compound is typically an α,β-unsaturated carbonyl compound: it contains a carbon–carbon double bond and a carbon–oxygen double bond (carbonyl) separated by a carbon–carbon single bond, thus possessing properties characteristic for both functional groups :
- at the C=C bond: electrophilic addition of acids and halogens, hydrogenation, hydroxylation and cleavage of the bond
- at the C=O bond: nucleophilic substitution (such as in esters) or nucleophilic addition (such as in ketones). The carboxyl group of acrylic acid can react with ammonia to form acrylamide, or with an alcohol to form an acrylate ester.
In addition, since both double bonds are separated by a single C–C bond, the double bonds are conjugated.
See also
This article is issued from Wikipedia - version of the 12/4/2015. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.