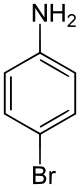
4-Bromoaniline
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
4-Bromoaniline | |||
| Other names
(4-Bromophenyl)amine p-Bromoaniline 4-Bromobenzenamine p-Bromophenylamine | |||
| Identifiers | |||
| 106-40-1 | |||
| 3D model (Jmol) | Interactive image | ||
| ChEMBL | ChEMBL57376 | ||
| ChemSpider | 7519 | ||
| ECHA InfoCard | 100.003.086 | ||
| EC Number | 203-393-9 | ||
| UNII | 0RR61TC330 | ||
| |||
| |||
| Properties | |||
| C6H6BrN | |||
| Molar mass | 172.02 g mol−1 | ||
| Density | 1.5 g/cm3 | ||
| Melting point | 60 to 64 °C (140 to 147 °F; 333 to 337 K) | ||
| <0.1 g/100 mL at 23 °C | |||
| Hazards | |||
| Safety data sheet | External MSDS | ||
| R-phrases | R20/21/22 R36/37/38 | ||
| S-phrases | S26 S36/37/39 | ||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| | |||
| Infobox references | |||
4-Bromoaniline is a compound where an aniline molecule is substituted with a bromine atom. Commercially available, this compound may be used as a building block, e.g. in the preparation of p-bromobiphenyl via the Gomberg-Bachmann reaction.[2]
References
- ↑ 4-Bromoaniline, Chemblink.com
- ↑ M. Gomberg and W. E. Bachmann (1941). "p-Bromobiphenyl". Org. Synth.; Coll. Vol., 1, p. 113
This article is issued from Wikipedia - version of the 9/4/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.