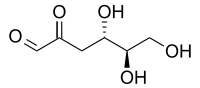
3-Deoxyglucosone
 | |
| Names | |
|---|---|
| IUPAC name
(4S,5R)-4,5,6-Trihydroxy-2-oxohexanal | |
| Other names
3-Deoxy-D-erythro-hexosulose; 2-Keto-3-deoxyglucose; 3-Deoxy-D-erythro-hexos-2-ulose; 3-Deoxy-D-erythro-hexosulose; 3-Deoxy-D-glucosone; D-3-Deoxyglucosone | |
| Identifiers | |
| 4084-27-9 | |
| 3D model (Jmol) | Interactive image Interactive image |
| ChEBI | CHEBI:60777 |
| ChemSpider | 102799 |
| PubChem | 114839 |
| |
| |
| Properties | |
| C6H10O5 | |
| Molar mass | 162.14 g·mol−1 |
| Density | 1.406 g/ml |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
3-Deoxyglucosone (3DG) is a dicarbonyl sugar that is synthesized through the Maillard reaction,[1] and is metabolized to 3-deoxyfructose and 2-keto-3-deoxygluconic acid. 3DG is a precursor for the formation of advanced glycation end-products (AGEs): 3DG rapidly reacts with protein amino groups to form AGEs such as imidazolone, pyrraline, N6-(carboxymethyl)lysine, and pentosidine. 3DG as well as AGEs play a role in the modification and cross-linking of long-lived proteins such as crystallin[2] and collagen,[3] contributing to diseases such as the vascular complications of diabetes, atherosclerosis, hypertension, Alzheimer's disease, inflammation, and aging.
Sources
3DG is a highly reactive sugar that is found in high-fructose corn syrup and in many foods. It is also made naturally by the body when excessive sugar is consumed or when a person is diabetic. Glucose reacts non-enzymatically with protein amino groups to initiate glycation, the early stage of the Maillard reaction. In the intermediate and late stages of glycation, the spontaneous formation of highly reactive compounds such as 3DG may account for the numerous features of diabetic complication as well as aging.
In 1990, the Brown group from Fox Chase Cancer Center in Philadelphia identified fructose 3-phosphate (F3P) in lenses from diabetic rats.[4] This shows the existence of the only mammalian kinase that phosphorylates a sugar on a secondary hydroxyl group. F3P is an unstable compound, and the spontaneous decomposition of F3P leads to formation of 3DG. In 2000, the 3-phosphokinase responsible for the formation of F3P was cloned and named fructosamine 3-kinase (FN3K).[5]
Biological activity and clinical implication
Emerging data indicate that 3DG plays a central role in the development of diabetic complications via FN3K action. 3DG has a variety of potential biological effects, particularly when it is present at elevated concentrations in diabetic states:
- Diabetic humans have elevated levels of 3DG and 3-deoxyfructose (3DF) in plasma and urine as compared with non-diabetic individuals. Development of diabetic complications is accelerated in patients with extremely high levels of 3DG in their serum. Diabetics with nephropathy were found to have elevated plasma levels of 3DG compared with other diabetics.[6][7][8]
- Glycated diet, which elevates systemic 3DG levels, leads to diabetes-like tubular and glomerular kidney pathology[9] and increased oxidative stress. Diabetic humans also show increased oxidative stress.[10]
- Increased 3DG is correlated to increased glomerular basement membrane width.[11]
- Aminoguanidine (AG), an agent that detoxifies 3DG pharmacologically via formation of rapidly excreted covalent derivatives,[12] has been shown to reduce AGE associated retinal, neural, arterial, and renal pathologies in animal models.[13][14][15][16] The problem with AG is that it is toxic in the quantities needed for efficacy.
- 3DG induces reactive oxygen species (ROS) that contribute to the development of diabetic complications.[17] Specifically, 3DG induces heparin-binding epidermal growth factor, a smooth muscle mitogen that is abundant in atherosclerotic plaques. This observation suggests that an increase in 3DG may trigger atherogenesis in diabetes.[18][19]
- 3DG inactivates some of the most important enzymes that protect cells from ROS. For example, glutathione peroxidase, a central antioxidant enzyme that uses glutathione to remove ROS, and glutathione reductase, which regenerates glutathione, are both inactivated by 3DG.[20][21]
- 3DG inactivates aldehyde reductase.[22] Aldehyde reductase is the cellular enzyme that protects the body from 3DG. Detoxification of 3DG to 3-deoxyfructose (3DF) is impaired in diabetic humans since their ratio of 3DG to 3DF in urine and plasma differs significantly from non-diabetic individuals.[23]
- 3DG induces ROS, resulting in oxidative DNA damage.[24] 3DG can be internalized by cells and internalized 3DG is responsible for the production of intracellular oxidative stress.[25]
- 3DG is a teratogenic factor in diabetic embryopathy, leading to embryo malformation.[26] This appears to arise from 3DG accumulation, which leads to superoxide-mediated embryopathy. Women with pre-existing diabetes or severe diabetes that develops during pregnancy are between 3 and 4 times more likely than other women to give birth to infants with birth defects.
- 3DG induces apoptosis in macrophage-derived cell lines[27] and is toxic to cultured cortical neurons[28] and PC12 cells.[29] A recent study on the cause of amyotropic lateral sclerosis, a form of motor neuron disease, has suggested that accumulation of 3DG can lead to neurotoxicity because of ROS generation.[30]
- 3DG glycates and crosslinks proteins leading to a complex mixture of compounds called advanced glycation end-products (AGEs).[31][32] AGEs have been postulated to contribute to the development of a range of diabetic complications including nephropathy, retinopathy, and neuropathy.[33] Elevated levels of 3DG-modified proteins are found in diabetic versus control rat kidneys.[34] In hyperglycemia, production of 3DG provides an amplification loop to sustain AGE generation, oxidative stress, and vascular activation.[35]
- Hemoglobin-AGE levels are elevated in diabetic individuals[36] and other AGE proteins have been shown in experimental models to accumulate with time, increasing from 5-50 fold over periods of 5–20 weeks in the retina, lens and renal cortex of diabetic rats. The inhibition of AGE formation reduced the extent of nephropathy in diabetic rats.[37] Therefore, substances that inhibit AGE formation may limit the progression of disease and may offer new tools for therapeutic interventions in the therapy of AGE-mediated disease.[38][39]
- AGEs have specific cellular receptors; the best-characterized are those called RAGE. The activation of cellular RAGE on endothelium, mononuclear phagocytes, and lymphocytes triggers the generation of free radicals and the expression of inflammatory gene mediators.[40] Such increases in oxidative stress lead to the activation of the transcription factor NF-κB and promote the expression of NF-κB regulated genes that have been associated with atherosclerosis.[38]
References
- ↑ L.C.Maillard (1912). "Action des acides aminés sur les sucres: formation des mélanoïdines par voie méthodique". Compte-rendu de l'Académie des sciences. 154: 66–68.
- ↑ Monnier VM, Cerami A (January 1981). "Nonenzymatic browning in vivo: possible process for aging of long-lived proteins". Science. 211 (4481): 491–3. doi:10.1126/science.6779377. PMID 6779377.
- ↑ Monnier VM, Kohn RR, Cerami A (January 1984). "Accelerated age-related browning of human collagen in diabetes mellitus". Proceedings of the National Academy of Sciences of the United States of America. 81 (2): 583–7. doi:10.1073/pnas.81.2.583. PMC 344723
 . PMID 6582514.
. PMID 6582514. - ↑ Szwergold BS, Kappler F, Brown TR (January 1990). "Identification of fructose 3-phosphate in the lens of diabetic rats". Science. 247 (4941): 451–4. doi:10.1126/science.2300805. PMID 2300805.
- ↑ Delpierre G, Rider MH, Collard F, Stroobant V, Vanstapel F, Santos H, Van Schaftingen E (October 2000). "Identification, cloning, and heterologous expression of a mammalian fructosamine-3-kinase". Diabetes. 49 (10): 1627–34. doi:10.2337/diabetes.49.10.1627. PMID 11016445.
- ↑ Kusunoki H, Miyata S, Ohara T, Liu BF, Uriuhara A, Kojima H, Suzuki K, Miyazaki H, Yamashita Y, Inaba K, Kasuga M (June 2003). "Relation between serum 3-deoxyglucosone and development of diabetic microangiopathy". Diabetes Care. 26 (6): 1889–94. doi:10.2337/diacare.26.6.1889. PMID 12766129.
- ↑ Niwa T, Takeda N, Yoshizumi H, Tatematsu A, Ohara M, Tomiyama S, Niimura K (October 1993). "Presence of 3-deoxyglucosone, a potent protein crosslinking intermediate of Maillard reaction, in diabetic serum". Biochemical and Biophysical Research Communications. 196 (2): 837–43. doi:10.1006/bbrc.1993.2325. PMID 8240359.
- ↑ Wells-Knecht KJ, Lyons TJ, McCance DR, Thorpe SR, Feather MS, Baynes JW (September 1994). "3-Deoxyfructose concentrations are increased in human plasma and urine in diabetes". Diabetes. 43 (9): 1152–6. doi:10.2337/diabetes.43.9.1152. PMID 8070616.
- ↑ Kappler F, Schwartz ML, Su B, Tobia AM, Brown T (2001). "DYN 12, a small molecule inhibitor of the enzyme amadorase, lowers plasma 3-deoxyglucosone levels in diabetic rats". Diabetes Technology & Therapeutics. 3 (4): 609–16. doi:10.1089/15209150152811234. PMID 11911173.
- ↑ Feillet-Coudray C, Choné F, Michel F, Rock E, Thiéblot P, Rayssiguier Y, Tauveron I, Mazur A (October 2002). "Divergence in plasmatic and urinary isoprostane levels in type 2 diabetes". Clinica Chimica Acta; International Journal of Clinical Chemistry. 324 (1-2): 25–30. doi:10.1016/S0009-8981(02)00213-9. PMID 12204421.
- ↑ Beisswenger PJ, Drummond KS, Nelson RG, Howell SK, Szwergold BS, Mauer M (November 2005). "Susceptibility to diabetic nephropathy is related to dicarbonyl and oxidative stress". Diabetes. 54 (11): 3274–81. doi:10.2337/diabetes.54.11.3274. PMID 16249455.
- ↑ Brownlee M (June 1994). "Lilly Lecture 1993. Glycation and diabetic complications". Diabetes. 43 (6): 836–41. doi:10.2337/diab.43.6.836. PMID 8194672.
- ↑ Ellis EN, Good BH (October 1991). "Prevention of glomerular basement membrane thickening by aminoguanidine in experimental diabetes mellitus". Metabolism. 40 (10): 1016–9. doi:10.1016/0026-0495(91)90122-D. PMID 1943726.
- ↑ Soulis-Liparota T, Cooper M, Papazoglou D, Clarke B, Jerums G (October 1991). "Retardation by aminoguanidine of development of albuminuria, mesangial expansion, and tissue fluorescence in streptozocin-induced diabetic rat". Diabetes. 40 (10): 1328–34. doi:10.2337/diabetes.40.10.1328. PMID 1834497.
- ↑ Edelstein D, Brownlee M (January 1992). "Aminoguanidine ameliorates albuminuria in diabetic hypertensive rats". Diabetologia. 35 (1): 96–7. doi:10.1007/BF00400859. PMID 1541387.
- ↑ Brownlee M, Vlassara H, Kooney A, Ulrich P, Cerami A (June 1986). "Aminoguanidine prevents diabetes-induced arterial wall protein cross-linking". Science. 232 (4758): 1629–32. doi:10.1126/science.3487117. PMID 3487117.
- ↑ Araki A (September 1997). "[Oxidative stress and diabetes mellitus: a possible role of alpha-dicarbonyl compounds in free radical formation]". Nihon Ronen Igakkai Zasshi. Japanese Journal of Geriatrics. 34 (9): 716–20. PMID 9430981.
- ↑ Taniguchi N, Kaneto H, Asahi M, Takahashi M, Wenyi C, Higashiyama S, Fujii J, Suzuki K, Kayanoki Y (July 1996). "Involvement of glycation and oxidative stress in diabetic macroangiopathy". Diabetes. 45 Suppl 3: S81–3. doi:10.2337/diab.45.3.s81. PMID 8674900.
- ↑ Che W, Asahi M, Takahashi M, Kaneto H, Okado A, Higashiyama S, Taniguchi N (July 1997). "Selective induction of heparin-binding epidermal growth factor-like growth factor by methylglyoxal and 3-deoxyglucosone in rat aortic smooth muscle cells. The involvement of reactive oxygen species formation and a possible implication for atherogenesis in diabetes". The Journal of Biological Chemistry. 272 (29): 18453–9. doi:10.1074/jbc.272.29.18453. PMID 9218489.
- ↑ Vander Jagt DL, Hunsaker LA, Vander Jagt TJ, Gomez MS, Gonzales DM, Deck LM, Royer RE (April 1997). "Inactivation of glutathione reductase by 4-hydroxynonenal and other endogenous aldehydes". Biochemical Pharmacology. 53 (8): 1133–40. doi:10.1016/S0006-2952(97)00090-7. PMID 9175718.
- ↑ Niwa T, Tsukushi S (February 2001). "3-deoxyglucosone and AGEs in uremic complications: inactivation of glutathione peroxidase by 3-deoxyglucosone". Kidney International. Supplement. 78: S37–41. doi:10.1046/j.1523-1755.2001.59780037.x. PMID 11168980.
- ↑ Takahashi M, Lu YB, Myint T, Fujii J, Wada Y, Taniguchi N (January 1995). "In vivo glycation of aldehyde reductase, a major 3-deoxyglucosone reducing enzyme: identification of glycation sites". Biochemistry. 34 (4): 1433–8. doi:10.1021/bi00004a038. PMID 7827091.
- ↑ Lal S, Kappler F, Walker M, Orchard TJ, Beisswenger PJ, Szwergold BS, Brown TR (June 1997). "Quantitation of 3-deoxyglucosone levels in human plasma". Archives of Biochemistry and Biophysics. 342 (2): 254–60. doi:10.1006/abbi.1997.0117. PMID 9186486.
- ↑ Shimoi K, Okitsu A, Green MH, Lowe JE, Ohta T, Kaji K, Terato H, Ide H, Kinae N (September 2001). "Oxidative DNA damage induced by high glucose and its suppression in human umbilical vein endothelial cells". Mutation Research. 480-481: 371–8. doi:10.1016/S0027-5107(01)00196-8. PMID 11506829.
- ↑ Sakiyama H, Takahashi M, Yamamoto T, Teshima T, Lee SH, Miyamoto Y, Misonou Y, Taniguchi N (February 2006). "The internalization and metabolism of 3-deoxyglucosone in human umbilical vein endothelial cells". Journal of Biochemistry. 139 (2): 245–53. doi:10.1093/jb/mvj017. PMID 16452312.
- ↑ Eriksson UJ, Wentzel P, Minhas HS, Thornalley PJ (December 1998). "Teratogenicity of 3-deoxyglucosone and diabetic embryopathy". Diabetes. 47 (12): 1960–6. doi:10.2337/diabetes.47.12.1960. PMID 9836531.
- ↑ Okado A, Kawasaki Y, Hasuike Y, Takahashi M, Teshima T, Fujii J, Taniguchi N (August 1996). "Induction of apoptotic cell death by methylglyoxal and 3-deoxyglucosone in macrophage-derived cell lines". Biochemical and Biophysical Research Communications. 225 (1): 219–24. doi:10.1006/bbrc.1996.1157. PMID 8769121.
- ↑ Kikuchi S, Shinpo K, Moriwaka F, Makita Z, Miyata T, Tashiro K (July 1999). "Neurotoxicity of methylglyoxal and 3-deoxyglucosone on cultured cortical neurons: synergism between glycation and oxidative stress, possibly involved in neurodegenerative diseases". Journal of Neuroscience Research. 57 (2): 280–9. doi:10.1002/(SICI)1097-4547(19990715)57:2<280::AID-JNR14>3.0.CO;2-U. PMID 10398306.
- ↑ Suzuki K, Koh YH, Mizuno H, Hamaoka R, Taniguchi N (February 1998). "Overexpression of aldehyde reductase protects PC12 cells from the cytotoxicity of methylglyoxal or 3-deoxyglucosone". Journal of Biochemistry. 123 (2): 353–7. doi:10.1093/oxfordjournals.jbchem.a021944. PMID 9538214.
- ↑ Shinpo K, Kikuchi S, Sasaki H, Ogata A, Moriwaka F, Tashiro K (April 2000). "Selective vulnerability of spinal motor neurons to reactive dicarbonyl compounds, intermediate products of glycation, in vitro: implication of inefficient glutathione system in spinal motor neurons". Brain Research. 861 (1): 151–9. doi:10.1016/S0006-8993(00)02047-3. PMID 10751575.
- ↑ Baynes JW, Thorpe SR & Murtiashaw MH (1984). "Nonenzymatic glucosylation of lysine residues in albumin". Methods in Enzymology. Methods in Enzymology. 106: 88–98. doi:10.1016/0076-6879(84)06010-9. ISBN 978-0-12-182006-0. PMID 6436646.
- ↑ Dyer DG, Blackledge JA, Thorpe SR, Baynes JW (June 1991). "Formation of pentosidine during nonenzymatic browning of proteins by glucose. Identification of glucose and other carbohydrates as possible precursors of pentosidine in vivo". The Journal of Biological Chemistry. 266 (18): 11654–60. PMID 1904867.
- ↑ Rahbar S, Kumar Yernini K, Scott S, Gonzales N, Lalezari I (September 1999). "Novel inhibitors of advanced glycation endproducts". Biochemical and Biophysical Research Communications. 262 (3): 651–6. doi:10.1006/bbrc.1999.1275. PMID 10471380.
- ↑ Niwa T, Katsuzaki T, Miyazaki S, Miyazaki T, Ishizaki Y, Hayase F, Tatemichi N, Takei Y (March 1997). "Immunohistochemical detection of imidazolone, a novel advanced glycation end product, in kidneys and aortas of diabetic patients". The Journal of Clinical Investigation. 99 (6): 1272–80. doi:10.1172/JCI119285. PMC 507942
 . PMID 9077536.
. PMID 9077536. - ↑ Yan SF, Ramasamy R, Naka Y, Schmidt AM (December 2003). "Glycation, inflammation, and RAGE: a scaffold for the macrovascular complications of diabetes and beyond". Circulation Research. 93 (12): 1159–69. doi:10.1161/01.RES.0000103862.26506.3D. PMID 14670831.
- ↑ Kostolanská J, Jakus V, Barák L (May 2009). "HbA1c and serum levels of advanced glycation and oxidation protein products in poorly and well controlled children and adolescents with type 1 diabetes mellitus". Journal of Pediatric Endocrinology & Metabolism. 22 (5): 433–42. doi:10.1515/JPEM.2009.22.5.433. PMID 19618662.
- ↑ Ninomiya, T.; et al. (2001). "A novel AGE production inhibitor, prevents progression of diabetic nephropathy in STZ-induced rats". Diabetes. 50 Suppl. (2): A178–179.
- 1 2 Bierhaus A, Hofmann MA, Ziegler R, Nawroth PP (March 1998). "AGEs and their interaction with AGE-receptors in vascular disease and diabetes mellitus. I. The AGE concept". Cardiovascular Research. 37 (3): 586–600. doi:10.1016/S0008-6363(97)00233-2. PMID 9659442.
- ↑ Thornalley, P.J. (1996). "Advanced glycation and the development of diabetic complications. Unifying the involvement of glucose, methylglyoxal and oxidative stress". Endocrinol. Metab. 3: 149–166.
- ↑ Hofmann MA, Drury S, Fu C, Qu W, Taguchi A, Lu Y, Avila C, Kambham N, Bierhaus A, Nawroth P, Neurath MF, Slattery T, Beach D, McClary J, Nagashima M, Morser J, Stern D, Schmidt AM (June 1999). "RAGE mediates a novel proinflammatory axis: a central cell surface receptor for S100/calgranulin polypeptides". Cell. 97 (7): 889–901. doi:10.1016/S0092-8674(00)80801-6. PMID 10399917.