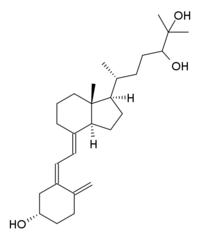
24,25-Dihydroxycholecalciferol
 | |
| Names | |
|---|---|
| IUPAC name
(6R)-6-[(1R,3aS,4E,7aR)-4-[(2Z)-2-[(5S)-5-hydroxy-2-methylenecyclohexylidene]ethylidene]-7a-methyl-2,3,3a,5,6,7-hexahydro-1H-inden-1-yl]-2-methylheptane-2,3-diol | |
| Other names
24,25-dihydroxyvitamin D3 (24R)-hydroxycalcifediol (24R)-hydroxycalcidiol | |
| Identifiers | |
| 40013-87-4 | |
| 3D model (Jmol) | Interactive image |
| ChEMBL | ChEMBL47183 |
| ChemSpider | 4939193 |
| ECHA InfoCard | 100.049.754 |
| PubChem | 6434253 |
| |
| |
| Properties | |
| C27H44O3 | |
| Molar mass | 416.63 g/mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
24,25-Dihydroxycholecalciferol, also known as 24,25-dihydroxyvitamin D3 and (24R)-hydroxycalcidiol (abbreviated as 24(R),25-(OH)2D3),[1] is a compound which is closely related to 1,25-dihydroxyvitamin D3, the active form of vitamin D3, but like vitamin D3 itself and 25-hydroxyvitamin D3 is inactive as a hormone both in vitro[2] and in vivo.[3] It was identified by Michael F. Holick.[4]
Formation and significance
24,25-dihydroxyvitamin D3 is formed from 25-hydroxyvitamin D3 by the action of P450cc24 (25-hydroxyvitamin D3-24-hydroxylase), which appears to be "a multicatalytic enzyme catalyzing most, if not all, of the reactions in the C-24/C-23 pathway of 25-OH-D3 metabolism."[5] It has been proposed that 24,25-dihydroxyvitamin D3 is a metabolite of 25-hydroxyvitamin D3 which is destined for excretion.[5]
It is not known whether the compound might also have some physiologically significant activity. Some evidence of a possible receptor has been obtained.[6]
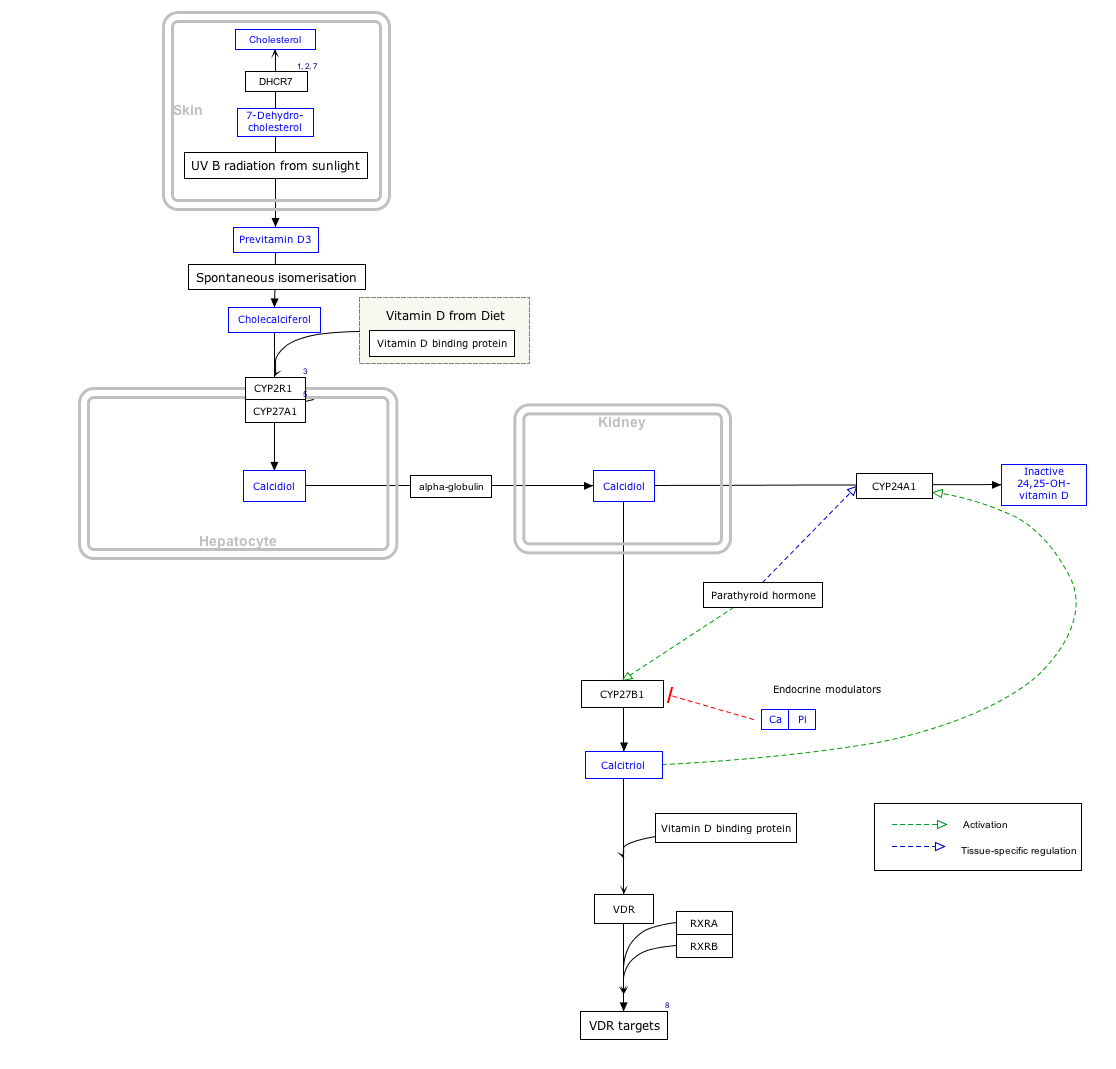
Interactive pathway map
Click on genes, proteins and metabolites below to link to respective articles. [§ 1]
Vitamin D Synthesis Pathway edit
- ↑ The interactive pathway map can be edited at WikiPathways: "VitaminDSynthesis_WP1531".
References
- ↑ "Nomenclature of Vitamin D. Recommendations 1981. IUPAC-IUB Joint Commission on Biochemical Nomenclature (JCBN)" reproduced at the Queen Mary, University of London website. Retrieved 21 March 2010.
- ↑ Sørnes, S.; Bjøro, T.; Berg, J. P.; Torjesen, P. A.; Haug, E. (1994). "Calcitriol attenuates the basal and vasoactive intestinal peptide-stimulated cAMP production in prolactin-secreting rat pituitary (GH4C1) cells". Molecular and cellular endocrinology. 101 (1–2): 183–188. doi:10.1016/0303-7207(94)90233-x. PMID 9397951.
- ↑ Mortensen, B. M.; Gautvik, K. M.; Gordeladze, J. O. (1993). "Bone turnover in rats treated with 1,25-dihydroxyvitamin D3, 25-hydroxyvitamin D3 or 24,25-dihydroxyvitamin D3". Bioscience reports. 13 (1): 27–39. doi:10.1007/bf01138176. PMID 8392394.
- ↑ Holick, MF; Schnoes, HK; Deluca, HF; Gray, RW; Boyle, IT; Suda, T (1972). "Isolation and identification of 24,25-dihydroxycholecalciferol, a metabolite of vitamin D made in the kidney". Biochemistry. 11 (23): 4251–5. doi:10.1021/bi00773a009. PMID 4342902.
- 1 2 Beckman, M. J.; Tadikonda, P.; Werner, E.; Prahl, J.; Yamada, S.; Deluca, H. F. (1996). "Human 25-Hydroxyvitamin D3-24-Hydroxylase, a Multicatalytic Enzyme†". Biochemistry. 35 (25): 8465–8472. doi:10.1021/bi960658i. PMID 8679605.
- ↑ Sömjen, D.; Sömjen, G. J.; Weisman, Y.; Binderman, I. (1982). "Evidence for 24,25-dihydroxycholecalciferol receptors in long bones of newborn rats". The Biochemical Journal. 204 (1): 31–36. doi:10.1042/bj2040031. PMC 1158312
 . PMID 6981414.
. PMID 6981414.
Other articles
- Mata-Granados, J. M.; Luque De Castro, M. D.; Quesada Gomez, J. M. (2008). "Inappropriate serum levels of retinol, α-tocopherol, 25 hydroxyvitamin D3 and 24,25 dihydroxyvitamin D3 levels in healthy Spanish adults: Simultaneous assessment by HPLC". Clinical Biochemistry. 41 (9): 676–680. doi:10.1016/j.clinbiochem.2008.02.003. PMID 18313404.