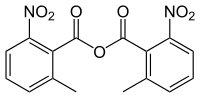
2-Methyl-6-nitrobenzoic anhydride
 | |
| Names | |
|---|---|
| IUPAC name
(2-Methyl-6-nitrobenzoyl) 2-methyl-6-nitrobenzoate | |
| Identifiers | |
| 434935-69-0 | |
| 3D model (Jmol) | Interactive image |
| ChemSpider | 8648059 |
| ECHA InfoCard | 100.156.789 |
| PubChem | 10472648 |
| |
| |
| Properties | |
| C16H12N2O7 | |
| Molar mass | 344.28 g·mol−1 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
2-Methyl-6-nitrobenzoic anhydride is a condensing agent also known as the Shiina reagent, having a structure wherein carboxylic acids undergo intermolecular dehydration condensation. It was developed in 2002 by Prof. Isamu Shiina (Tokyo University of Science, Japan).[1] The compound is often abbreviated MNBA.
Abstract
The reagent is used for synthetic reactions wherein medium- and large-sized lactones are formed from hydroxycarboxylic acids via intramolecular ring closure (Shiina macrolactonization).[2][3] The reaction proceeds at room temperature under basic or neutral conditions. This reagent can be used not only for macrolactonization but also for esterification, amidation, and peptide coupling.
See also
- Condensation reaction
- Shiina macrolactonization
- Shiina esterification
- Fischer-Speier esterification
- Steglich esterification
- Yamaguchi esterification
- Mitsunobu reaction
References
- ↑ Shiina, I.; Ibuka, R.; Kubota, M. (2002). "A New Condensation Reaction for the Synthesis of Carboxylic Esters from Nearly Equimolar Amounts of Carboxylic Acids and Alcohols Using 2-Methyl-6-nitrobenzoic Anhydride". Chem. Lett. 31 (3): 286. doi:10.1246/cl.2002.286.
- ↑ Shiina, I.; Kubota, M.; Oshiumi, H.; Hashizume, M. (2004). "An Effective Use of Benzoic Anhydride and Its Derivatives for the Synthesis of Carboxylic Esters and Lactones: A Powerful and Convenient Mixed Anhydride Method Promoted by Basic Catalysts". J. Org. Chem. 69 (6): 1822. doi:10.1021/jo030367x.
- ↑ Shiina, I. (2014). "An Adventurous Synthetic Journey with MNBA from Its Reaction Chemistry to the Total Synthesis of Natural Products". Bull. Chem. Soc. Jpn. 87 (2): 196. doi:10.1246/bcsj.20130216.
External links
- Enantioselective Total Synthesis of Octalactin A Using Asymmetric Aldol Reactions and a Rapid Lactonization To Form a Medium-Sized Ring
- Total Synthesis of Iejimalide B. An Application of the Shiina Macrolactonization
This article is issued from Wikipedia - version of the 7/27/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.