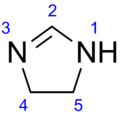
2-Imidazoline
 | |
 | |
| Names | |
|---|---|
| IUPAC name
4,5-Dihydro-1H-imidazole | |
| Identifiers | |
| 504-75-6 | |
| 3D model (Jmol) | Interactive image |
| ChEBI | CHEBI:53094 |
| ChemSpider | 61464 |
| ECHA InfoCard | 100.007.273 |
| PubChem | 68156 |
| |
| |
| Properties | |
| C3H6N2 | |
| Molar mass | 70.10 g·mol−1 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
2-Imidazoline (dihydroimidazoles) is one of three isomers of the nitrogen-containing heterocycle imidazoline, with the formula C3H6N2. The 2-imidazolines are the most common imidazolines commercially, as the ring exists in some natural products and some pharmaceuticals. They also have been examined in the context of organic synthesis, coordination chemistry, and homogeneous catalysis.[1]
Synthesis
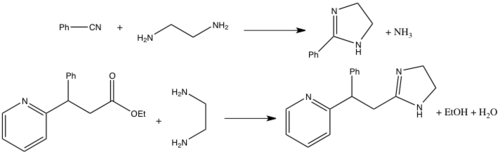
A variety of routes exist for the synthesis of imidazolines,[1][2] with the most common methods involving the condensation of 1,2-diamines (e.g. ethylenediamine) with nitriles or esters. The nitrile based route essentially a cyclic Pinner reaction; it requires high temperatures and acid catalysis and is effective for both alkyl and aryl nitriles.
Biological role
Many imidazolines are biologically active.[3] Most bio-active derivatives bear a substituent (aryl or alkyl group) on the carbon between the nitrogen centers. Some generic names include oxymetazoline, xylometazoline, tetrahydrozoline, and naphazoline.
Imidazoline in Natural Products
Imidazoline has been found in various natural products. Natural molecules topsentin D and spongotine B were discovered in several marine sponges. These metabolites have received considerable attention because of their potent properties such as antitumor, antiviral, and anti-inflammatory activities.[4]
Pharmaceutical Applications
2-imidazolines have been investigated as antihyperglycemic, anti-inflammatory, antihypertensive, antihypercholesterolemic, and antidepressant reagents.[1][5] The imidazoline-containing drug clonidine is used alone or in combination with other medications to treat high blood pressure. It is also used in the treatment of dysmenorrhea, hypertensive crisis, Tourette's syndrome and attention deficit hyperactivity disorder (ADHD).[6]
- 2-Imidazolines
-
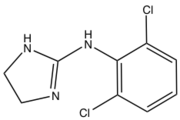
Clonidine
-
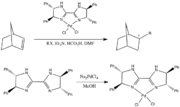
Biimidazoline ligands and a complex.
-

Second generation Grubbs' catalyst
-
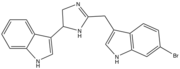
Spongotine B
Applications
Homogeneous Catalysis
As a structural analogue of 2-oxazolines, 2-imidazolines have been developed as ligands in coordination chemistry. The substitutions on the nitrogen atom in the imidazoline ring provide opportunities for fine-tuning the electronic and steric properties. Some of the complexes function as catalysts for Suzuki–Miyaura couplings, Mizoroki–Heck reactions, Diels–Alder reactions, asymmetric allylic substitution, [3,3] sigmatropic rearrangement, Henry reactions, etc.[1]
Surfactants
Surfactants based around 2-imidazoline, such as sodium lauroamphoacetate, are used in personal care products were mildness and non-irritancy are particularly important (e.g. baby products, "no more tears" shampoos etc).[7]
Imidazolines as Precursors of Imidazoles
Imidazoles can be prepared from dehydrogenation of imidazolines.[8]

See also
References
- 1 2 3 4 Liu, H.; Du, D.-M. (2009). "Recent Advances in the Synthesis of 2-Imidazolines and Their Applications in Homogeneous Catalysis". Adv. Synth. Catal. 351: 489–519. doi:10.1002/adsc.200800797.
- ↑ David Crouch, R. (March 2009). "Synthetic routes toward 2-substituted 2-imidazolines". Tetrahedron. 65 (12): 2387–2397. doi:10.1016/j.tet.2008.12.022.
- ↑ N. MacInnes and S. Duty (2004). "Locomotor effects of imidazoline I2-site-specific ligands and monoamine oxidase inhibitors in rats with a unilateral 6-hydroxydopamine lesion of the nigrostriatal pathway". Br J Pharmacol. 143 (8): 952–959. doi:10.1038/sj.bjp.0706019. PMC 1575965
 . PMID 15545290.
. PMID 15545290. - ↑ Guinchard, X.; Valle; Denis, J. N. (2007). "Total Synthesis of Marine Sponge Bis(indole) Alkaloids of the Topsentin Class". J. Org. Chem. 72 (10): 3972–3975. doi:10.1021/jo070286r.
- ↑ Dardonville, C.; Rozas, I. (2004). "Imidazoline binding sites and their ligands: An overview of the different chemical structures". Med. Res. Rev. 24: 639–661. doi:10.1002/med.20007.
- ↑ "Clonidine", Pubmed Health, http://www.ncbi.nlm.nih.gov/pubmedhealth/PMH0000623/
- ↑ Tyagi, R.; Tyagi, V. K.; Pandey, S. K. (2007). "Imidazoline and its derivatives: an overview". J. Oleo. Sci. 56: 211–222. doi:10.5650/jos.56.21.
- ↑ Ishihara, M.; Togo, H. (2006). "An Efficient Preparation of 2-Imidazolines and Imidazoles from Aldehydes with Molecular Iodine and (Diacetoxyiodo)benzene". Synlett: 227–230. doi:10.1055/s-2005-923604.