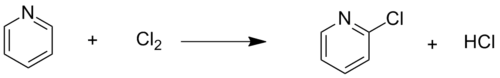2-Chloropyridine
 | |||
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
2-Chloropyridine | |||
| Identifiers | |||
| 109-09-1 | |||
| 3D model (Jmol) | Interactive image | ||
| ChEBI | CHEBI:39174 | ||
| ChEMBL | ChEMBL509579 | ||
| ChemSpider | 7689 | ||
| ECHA InfoCard | 100.003.316 | ||
| UNII | 8HMD45AYEJ | ||
| |||
| |||
| Properties | |||
| C5H4ClN | |||
| Molar mass | 113.54 g/mol | ||
| Appearance | colorless liquid | ||
| Melting point | −46 °C (−51 °F; 227 K) | ||
| Boiling point | 166 °C (331 °F; 439 K) | ||
| 27 g/L | |||
| Acidity (pKa) | 0.49 [1] | ||
| Hazards | |||
| Safety data sheet | MSDS | ||
| R-phrases | R23 R38 | ||
| S-phrases | S26 S37 S39 S45 | ||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| | |||
| Infobox references | |||
2-Chloropyridine is an organohalide with the formula C5H4ClN. It is a colorless liquid that is mainly used to generate fungicides and insecticides in industry. It also serves to generate antihistamines and antiarrythymics for pharmaceutical purposes.[2]
Preparation
2-Choropyridine was originally described in 1898 by the chlorination of 2-hydroxypyridine.[3] A typical chlorinating agent is phosphoryl chloride. It can also be generated by halogenating pyridine. This reaction affords a mixture of 2-chloro and 2,6-dichloropyridine.[2]
Alternatively, 2-chloropyridines can be conveniently synthesized in high yields from pyridine-N-oxides.[4]
Structure and properties
2-Chloropyridine reacts with nucleophiles to generate pyridine derivatives substituted at the second and fourth carbons on the heterocycle. Therefore, many reactions using 2-chloropyridine generate mixtures of products which require further workup to isolate the desired isomer.[2]
Main reactions and applications
2-chloropyridine is primarily used to generate other pyridine derivatives. Some commercial products include pyrithione, pyripropoxyfen, chlorphenamine, and disopyramide. These reactions rely on chloride’s nature as a good leaving group to facilitate the transfer of a substrate onto the pyridine ring.[2] Pyrithione, the conjugate base of 2-mercaptopyridine-N-oxide, is a fungicide found in some shampoos. It is generated from 2-chloropyridine by reacting the N-oxide of 2-chloropyridine with Na2S in a basic solution, before adding aqueous HCl.[5] Used as an antihistamine, pheniramine may be generated via several different pathways. One synthesis is to hydroformylate functionalized olefins. This reaction proceeds by reacting phenylacetonitrile with 2-chloropyridine in the presence of a base. The resulting intermediate is then alkylated by 2-(dimethylamino)ethyl chloride and the cyano group removed.[6]
Environmental Properties
Though pyridine is an excellent source of carbon, nitrogen, and energy for certain microorganisms, introduction of a halogen moiety significantly retards degradation of the pyridine ring. With the exception of 4-chloropyridine, each of the mono- and di-substituted chloropyridines were found to be relatively resistant to microbiological degradation in soil or liquid media.[7] Estimated time for complete degradation was > 30 days. 2-Chloropyridine exhibits extensive volatilization losses from water, less so when present in soil.[8]
References
- ↑ Linnell, R. H., J. Org. Chem., 1960, 25, 290.
- 1 2 3 4 Shimizu, Shinkichi et al. Pyridine and Pyridine Derivatives. Ullmann’s Encyclopedia of Industrial Chemistry. 2000. doi:10.1002/14356007.a22_399
- ↑ Sell, William J.; Dootson, Frederick W. The chlorine derivatives of pyridine. Part I. Journal of the Chemical Society, Transactions 1898, 73, pp. 432-441. http://www.rsc.org/ejarchive/CT/1898/CT8987300432.pdf
- ↑ P. Naender, B. Gangadasu, Chilukuri Ramesh, B.C. Raju and V.J. Rao. Facile and Selective Synthesis of Chloromethypyridines and Chloropyridines using Diphosgene/Triphosgene. Synthetic Communications. 34, 6, 1097, 2004
- ↑ Cheng, Hefeng; She, Ji. 14. Improved preparation of 2-mercaptopyridine-N-oxide. Zhongguo Yiyao Gongye Zazhi. 1990, 21, (2), pp. 55-56. ISSN 1001-8255
- ↑ Botteghi, Carlo et al. New Synthetic Route to Pheniramines via Hydroformylation of Functionalyzed Olefins. 1994, 59, pp. 7125-7127. doi:10.1021/jo00102a044
- ↑ Sims, G. K. and L.E. Sommers. 1986. Biodegradation of pyridine derivatives in soil suspensions. Environmental Toxicology and Chemistry. 5:503-509.
- ↑ Sims, G. K. and L.E. Sommers. 1985. Degradation of pyridine derivatives in soil. Journal of Environmental Quality. 14:580-584.