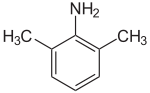
2,6-Xylidine
 | |
| Names | |
|---|---|
| IUPAC name
2,6-dimethylbenzene-1-amine | |
| Other names
2,6-dimethylaniline, 2,6-dimethylbenzenamine, 2,6-dimethylphenylamine | |
| Identifiers | |
| 87-62-7 | |
| 3D model (Jmol) | Interactive image |
| ChEBI | CHEBI:28738 |
| ChemSpider | 6630 |
| ECHA InfoCard | 100.001.599 |
| KEGG | C11004 |
| |
| |
| Properties | |
| C8H11N | |
| Molar mass | 121.18 g·mol−1 |
| Appearance | colourless liquid |
| Density | 0.9842 g/mL |
| Melting point | 11.45 °C (52.61 °F; 284.60 K) |
| Boiling point | 215 °C (419 °F; 488 K) |
| Refractive index (nD) |
1.5601 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
2,6-Xylidine is an aniline derivative with the chemical formula (CH3)2C6H3NH2. It is an isomer of 2,4-xylidine and 3,4-xylidine. It is a colourless liquid although commercial samples can appear to be yellow or even red.
Production and reactions
Many xylidines are prepared by nitration of a xylene followed by hydrogenation of the nitroaromatic, but this approach is not efficient for this isomer. Instead, it is prepared from by treatment of the related xylenol with ammonia in the presence of oxide catalysts.[1]
2,6-Xylidine is a precursor to the anesthetic lidocaine:[2] It is the precursor to the NHC ligand called Imes.[3]
References
- ↑ M. Meyer "Xylidines" in Ullmann's Encylclopedia of Industrial Chemistry Wiley-VCH, Weinheim, 2012. doi:10.1002/14356007.a28_455
- ↑ T. J. Reilly (1999). "The Preparation of Lidocaine". J. Chem. Ed. 76 (11): 1557. doi:10.1021/ed076p1557.
- ↑ Ison, E. A., Ison, A., "Synthesis of Well-Defined Copper N-Heterocyclic Carbene Complexes and Their Use as Catalysts for a "Click Reaction": A Multistep Experiment That Emphasizes the Role of Catalysis in Green Chemistry", Journal of Chemical Education 2012, volume 89, p. 1575. doi:10.1021/ed300243s
This article is issued from Wikipedia - version of the 3/26/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.
